Popular on TelAve
- Oklahoma and Starlink Local Installers getting it done! / now offering digital menu board installs - 124
- Starlink Local Installers working with state of Minnesota (now offering digital menu board installs) - 112
- Phinge CEO Ranked #1 Globally by Crunchbase for the Last Week, Will Be in Las Vegas Jan. 4-9, the Week of CES to Discuss Netverse & IPO Coming in 2026
- Starlink Local Installers helping Wisconsin stay wired (now offering digital menu board installs)
- BEC Technologies Expands MX-220 5G Industrial Router Series for Edge Connectivity
- Local Fiber Announces Graduation from Samsung Next Startup Program, Marks New Phase of Growth
- T-TECH Partners with Japan USA Precision Tools for 2026 US Market Development of the New T-TECH 5-Axis QUICK MILL™
- America's Most Festive Garages Wanted for Garage.com's 2025 Holiday Contest
- From Cheer to Courtroom: The Hidden Legal Risks in Your Holiday Eggnog
- Kaufman Development Breaks Ground on Detroit Micro Data Center, Expanding Its National AI Platform
Similar on TelAve
- Walmart $WMT and COSTCO.COM $COST Distribution as SonicShieldX™ Platform Sets the Stage for Accelerated Growth in 2026: AXIL Brands (N Y S E: AXIL)
- AI-Driven Drug Development with Publication of New Bioinformatics Whitepaper for BullFrog AI: $BFRG Strengthens Its Position in AI Drug Development
- IQSTEL Enters 2026 from a Position of Strength Following Transformational Year Marked by N A S D A Q Uplisting, Record Revenue and First-Ever
- Appliance EMT Expands Professional Appliance Repair Services to Hartford, Connecticut
- Java Holdings LLC Acquires +Peptide, Expanding Portfolio Across Coffee, Science, and Functional Nutrition
- OneSolution® Expands to Orlando with New Altamonte Springs Implant Center
- 2025: A Turning Point for Human Rights. CCHR Demands End to Coercive Psychiatry
- The 22% Tax Reality: Finland's New Gambling Law Creates a "Fiscal Trap" for Grey Market Casino Players
- UK Financial Ltd Executes Compliance Tasks Ahead Of First-Ever ERC-3643 Exchange-Traded Token, SMCAT & Sets Date For Online Investor Governance Vote
- HBZBZL Unveils "Intelligent Ecosystem" Strategy: Integrating AI Analytics with Web3 Incubation
Agreement for $27 Million in Funding for Expanding Clinic Acquisitions and Operations,: NRx Pharmaceuticals, Inc. Stock Symbol: NRXP)
TelAve News/10850461
$NRXP New Drug Application for Treatment of Suicidal Depression. Completion of NDA Filing Expected in First Quarter of 2025
MIAMI - TelAve -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Binding Term Sheet for $27 Million in Funding for HOPE Clinic Acquisitions and Pharmaceutical Operations
Kadima Neuropsychiatry Institute Targeted as First Acquisition for HOPE Subsidiary International Network of Interventional Psychiatry Clinics.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research.
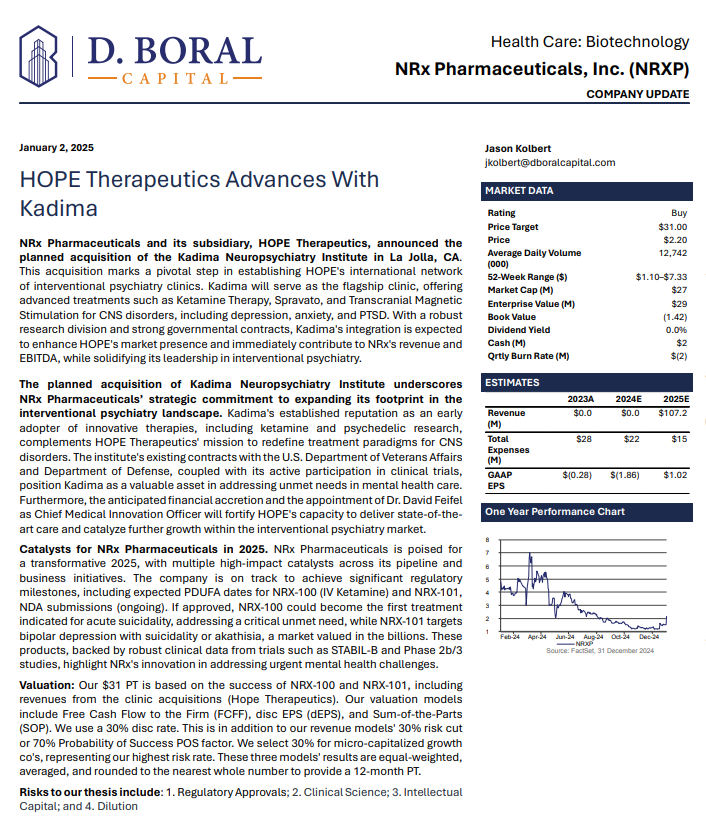
NRXP given a $31 Price Target from Respected Investment Analyst D. Boral Capital
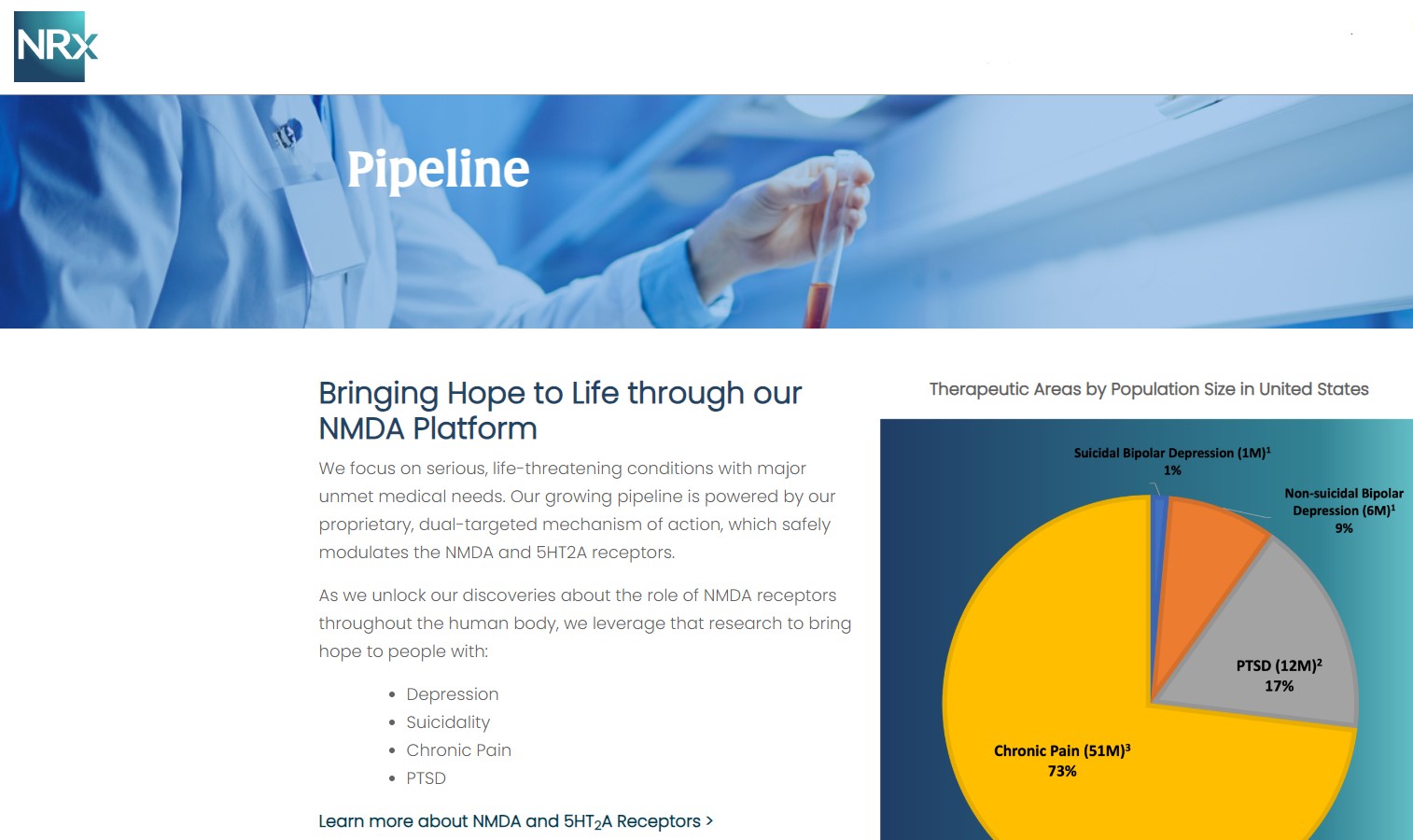
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
More on TelAve News
The new D. Boral valuation includes the following commentary: Our $31 PT is based on the success of NRX-100 and NRX-101, including revenues from the clinic acquisitions (Hope Therapeutics). Our valuation models include Free Cash Flow to the Firm (FCFF), disc EPS (dEPS), and Sum-of-the-Parts (SOP). We use a 30% disc rate. This is in addition to our revenue models' 30% risk cut or 70% Probability of Success POS factor. We select 30% for micro-capitalized growth co's, representing our highest risk rate. These three models' results are equal-weighted, averaged, and rounded to the nearest whole number to provide a 12-month PT.
Binding Term Sheet for $27 Million in Funding for HOPE Clinic Acquisitions and Pharmaceutical Operations
On January 6th NRXP announced signing of a Binding Term Sheet with Smith & Sauer, LLC, for $25 million equity purchases to fund planned HOPE clinic subsidiary acquisitions. Company management anticipates this new capital in combination with anticipated bank and/or bond lending to be sufficient to drive substantial revenue in HOPE clinic acquisition during 2025, as recently forecast by independent securities analysts. An additional $2 million equity investment in NRXP will be used to advance the Company's new drug applications for NRX-100 and NRX-101.
Benefits of this agreement include:
Anticipated capital from funds managed by Smith & Sauer to augment potential bank-financing for acquisition of HOPE Therapeutics clinics and support NRXP operations.
Purchase of $25 million in Series A Preferred Stock in HOPE Therapeutics (non-dilutive to NRXP shareholders) convertible into 1/3 of fully diluted HOPE Therapeutics equity, with a 15% current preferred dividend (non-callable for 2 years) for planned HOPE clinic acquisitions.
Purchase $2 million in NRXP equity, above the most recent closing price, at $2.75 per share. Investor will also purchase 500,000 NRXP shares from existing shareholders for $2.75 per share
In connection with both investments, investor shall receive warrants to purchase 3 million currently unregistered shares of NRXP common stock at $3.00 per share with a 24-month term.
Smith & Sauer will also be entitled to receive royalties on net revenues from NRXP product sales to a defined rate of return
Investment in HOPE Therapeutics is expected to enhance the NRXP balance sheet value, strengthening its financial position.
Smith & Sauer to join the HOPE Therapeutics and NRx Pharmaceuticals' Boards of Directors
Kadima Neuropsychiatry Institute Expected First Acquisition for HOPE Subsidiary International Network of Interventional Psychiatry Clinics
On January 2nd NRXP announced the planned acquisition of the Kadima Neuropsychiatry Institute of La Jolla, CA, per the previously announced Letter of Intent, for the Company's HOPE subsidiary network. Kadima is expected to serve as the flagship clinic for HOPE's planned international network of interventional psychiatry clinics, designed to provide advanced treatments for debilitating diseases such as depression, anxiety and PTSD.
More on TelAve News
Kadima is one of the world's premier interventional psychiatry clinics and was among the first to introduce Ketamine Therapy for Central Nervous System (CNS) disorders at scale in the clinic setting. The clinic offers a full range of cutting-edge treatments for suicidal depression, anxiety, Post Traumatic Stress Disorder (PTSD) and other CNS disorders. Those treatment options include Ketamine Therapy, Spravato® (nasal esketamine), Transcranial Magnetic Stimulation as well as medication management.
Kadima also has a robust research division and is a leading investigative site for innovative CNS treatments, specializing in psychedelic research, for which it has served as a leading site in nearly all major clinical trials in this area. Kadima has contracts in place with the US Department of Veterans Affairs and also treats active-duty military personnel in the US Department of Defense under Tricare and other treatment programs.
Kadima's founder and CEO, Prof. David Feifel, MD PHD, has been a pioneer and international thought leader for advanced interventional treatment of psychiatric disorders such as depression, anxiety, PTSD and related disorders for more than three decades.
Upon consummation of the acquisition, Dr. Feifel will serve as NRXP HOPE's Chief Medical Innovation Officer (CMIO), focused on identifying and evaluating new developments in the treatment of CNS disorders and insuring Hope clinics are at the forefront of interventional psychiatry delivery, and leading global clinical trials to continue to advance the ability to treat these lethal diseases.
Initial Section of U.S. New Drug Application to the FDA for NRX-100 (IV Ketamine) for the Treatment of Suicidal Depression
On December 30th NRXP announced the transmission of first section of its New Drug Application (NDA) for NRX-100 (ketamine) for electronic filing with the U.S. Food & Drug Administration (FDA). NRX-100 was initially granted Fast Track Designation in 2017 for use in combination with NRX-101 (D-cycloserine/lurasidone) for treatment of suicidal bipolar depression. The Company is now seeking to expand the indication to include Suicidal Ideation in Major Depressive Disorder and other forms of depression, based on data from NIH- and European Government-funded trials that have been summarized on the NRXP website.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Binding Term Sheet for $27 Million in Funding for HOPE Clinic Acquisitions and Pharmaceutical Operations
Kadima Neuropsychiatry Institute Targeted as First Acquisition for HOPE Subsidiary International Network of Interventional Psychiatry Clinics.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research.
NRXP given a $31 Price Target from Respected Investment Analyst D. Boral Capital
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
More on TelAve News
- Appliance EMT Expands Professional Appliance Repair Services to Hartford, Connecticut
- Java Holdings LLC Acquires +Peptide, Expanding Portfolio Across Coffee, Science, and Functional Nutrition
- OneSolution® Expands to Orlando with New Altamonte Springs Implant Center
- Indian Peaks Veterinary Hospital Launches Updated Dental Services Page for Boulder Pet Owners
- Dugan Air Donates $10,000 to Indian Creek Schools
The new D. Boral valuation includes the following commentary: Our $31 PT is based on the success of NRX-100 and NRX-101, including revenues from the clinic acquisitions (Hope Therapeutics). Our valuation models include Free Cash Flow to the Firm (FCFF), disc EPS (dEPS), and Sum-of-the-Parts (SOP). We use a 30% disc rate. This is in addition to our revenue models' 30% risk cut or 70% Probability of Success POS factor. We select 30% for micro-capitalized growth co's, representing our highest risk rate. These three models' results are equal-weighted, averaged, and rounded to the nearest whole number to provide a 12-month PT.
Binding Term Sheet for $27 Million in Funding for HOPE Clinic Acquisitions and Pharmaceutical Operations
On January 6th NRXP announced signing of a Binding Term Sheet with Smith & Sauer, LLC, for $25 million equity purchases to fund planned HOPE clinic subsidiary acquisitions. Company management anticipates this new capital in combination with anticipated bank and/or bond lending to be sufficient to drive substantial revenue in HOPE clinic acquisition during 2025, as recently forecast by independent securities analysts. An additional $2 million equity investment in NRXP will be used to advance the Company's new drug applications for NRX-100 and NRX-101.
Benefits of this agreement include:
Anticipated capital from funds managed by Smith & Sauer to augment potential bank-financing for acquisition of HOPE Therapeutics clinics and support NRXP operations.
Purchase of $25 million in Series A Preferred Stock in HOPE Therapeutics (non-dilutive to NRXP shareholders) convertible into 1/3 of fully diluted HOPE Therapeutics equity, with a 15% current preferred dividend (non-callable for 2 years) for planned HOPE clinic acquisitions.
Purchase $2 million in NRXP equity, above the most recent closing price, at $2.75 per share. Investor will also purchase 500,000 NRXP shares from existing shareholders for $2.75 per share
In connection with both investments, investor shall receive warrants to purchase 3 million currently unregistered shares of NRXP common stock at $3.00 per share with a 24-month term.
Smith & Sauer will also be entitled to receive royalties on net revenues from NRXP product sales to a defined rate of return
Investment in HOPE Therapeutics is expected to enhance the NRXP balance sheet value, strengthening its financial position.
Smith & Sauer to join the HOPE Therapeutics and NRx Pharmaceuticals' Boards of Directors
Kadima Neuropsychiatry Institute Expected First Acquisition for HOPE Subsidiary International Network of Interventional Psychiatry Clinics
On January 2nd NRXP announced the planned acquisition of the Kadima Neuropsychiatry Institute of La Jolla, CA, per the previously announced Letter of Intent, for the Company's HOPE subsidiary network. Kadima is expected to serve as the flagship clinic for HOPE's planned international network of interventional psychiatry clinics, designed to provide advanced treatments for debilitating diseases such as depression, anxiety and PTSD.
More on TelAve News
- Robert DeMaio, Phinge Founder & CEO, Ranked #1 Globally on Crunchbase, Continues to Convert Previous Debt Owed to Him by Phinge into Convertible Notes
- 2025: A Turning Point for Human Rights. CCHR Demands End to Coercive Psychiatry
- The 22% Tax Reality: Finland's New Gambling Law Creates a "Fiscal Trap" for Grey Market Casino Players
- Phinge Founder & CEO Robert DeMaio Ranked #1 Globally on Crunchbase, Continues to Convert Previous Debt Owed to Him by Phinge into Convertible Notes
- Donna Cardellino Manager/Facilitator Signs Justin Jeansonne Country Singer-Songwriter To Exclusive Management Deal For Global Music Expansion
Kadima is one of the world's premier interventional psychiatry clinics and was among the first to introduce Ketamine Therapy for Central Nervous System (CNS) disorders at scale in the clinic setting. The clinic offers a full range of cutting-edge treatments for suicidal depression, anxiety, Post Traumatic Stress Disorder (PTSD) and other CNS disorders. Those treatment options include Ketamine Therapy, Spravato® (nasal esketamine), Transcranial Magnetic Stimulation as well as medication management.
Kadima also has a robust research division and is a leading investigative site for innovative CNS treatments, specializing in psychedelic research, for which it has served as a leading site in nearly all major clinical trials in this area. Kadima has contracts in place with the US Department of Veterans Affairs and also treats active-duty military personnel in the US Department of Defense under Tricare and other treatment programs.
Kadima's founder and CEO, Prof. David Feifel, MD PHD, has been a pioneer and international thought leader for advanced interventional treatment of psychiatric disorders such as depression, anxiety, PTSD and related disorders for more than three decades.
Upon consummation of the acquisition, Dr. Feifel will serve as NRXP HOPE's Chief Medical Innovation Officer (CMIO), focused on identifying and evaluating new developments in the treatment of CNS disorders and insuring Hope clinics are at the forefront of interventional psychiatry delivery, and leading global clinical trials to continue to advance the ability to treat these lethal diseases.
Initial Section of U.S. New Drug Application to the FDA for NRX-100 (IV Ketamine) for the Treatment of Suicidal Depression
On December 30th NRXP announced the transmission of first section of its New Drug Application (NDA) for NRX-100 (ketamine) for electronic filing with the U.S. Food & Drug Administration (FDA). NRX-100 was initially granted Fast Track Designation in 2017 for use in combination with NRX-101 (D-cycloserine/lurasidone) for treatment of suicidal bipolar depression. The Company is now seeking to expand the indication to include Suicidal Ideation in Major Depressive Disorder and other forms of depression, based on data from NIH- and European Government-funded trials that have been summarized on the NRXP website.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on TelAve News
- Renowned Alternative Medicine Specialist Dr. Sebi and His African Bio Mineral Balance Therapy Are the Focus of New Book
- Psychiatric Drug Damage Ignored for Decades; CCHR Demands Federal Action
- Why Millions Are Losing Sexual Sensation, And Why It's Not Age, Hormones, or Desire
- Justin Jeansonne An Emerging Country Singer-Songwriter Music Fans Have Been Waiting For…a True Maverick
- Russellville Huntington Learning Center Expands Access to Literacy Support; Approved Provider Under Arkansas Department of Education
- UK Financial Ltd Launches U.S. Operations Following Delaware Approval
- Pinealage: the app that turns strangers into meditation companions — in crowdfunding phase
- "Micro-Studio": Why San Diegans are Swapping Crowded Gyms for Private, One-on-One Training at Sweat Society
- Beycome Closes $2.5M Seed Round Led by InsurTech Fund
- Tru by Hilton Columbia South Opens to Guests
- Christy Sports donates $56K in new gear to SOS Outreach to help kids hit the slopes
- "BigPirate" Sets Sail: A New Narrative-Driven Social Casino Adventure
- Phinge CEO Ranked #1 Globally by Crunchbase for the Last Week, Will Be in Las Vegas Jan. 4-9, the Week of CES to Discuss Netverse & IPO Coming in 2026
- Women's Everyday Safety Is Changing - The Blue Luna Shows How
- Microgaming Unveils Red Papaya: A New Studio Delivering Cutting-Edge, Feature-Rich Slots
- 5-Star Duncan Injury Group Expands Personal Injury Representation to Arizona
- The End of "Influencer" Gambling: Bonusetu Analyzes Finland's Strict New Casino Marketing Laws
- AI-Driven Cybersecurity Leader Gains Industry Recognition, Secures $6M Institutional Investment, Builds Momentum Toward $16M Annual Run-Rate Revenue
- TRIO Heating, Air & Plumbing Now Ranks #1 in San Jose
- Milwaukee Job Corps Center Hosts Alumni Day, Calls Alumni to Action on Open Enrollment Campaign