Popular on TelAve
- Althea Gibson Honored as Final Release in U.S. Mint's American Women Quarters Program - 156
- Cyntexa Announces Updates to ChargeOn on Salesforce AppExchange
- TradingHabits.com Launches to Support Day Trader Well-being
- 5,000 Australians Call for Clarity: NaturismRE's Petition Reaches Major Milestone
- BumblebeeSmart Introduces Rounded Busy Board Set for Preschoolers
- Wohler announces three SRT monitoring enhancements for its iVAM2-MPEG monitor and the addition of front panel PID selection of A/V/subtitle streams
- Dental Care Solutions Unveils New Website for Enhanced Patient Engagement
- WHES Retains BloombergNEF Tier 1 Ranking for Sixth Consecutive Quarter
- Huntington Learning Center of Russellville Marks 1 Year Anniversary; Extends Reduced Grant-Aligned Rates to All Students in Learning Center Services
- Vertical Consultants and Cell Tower AI™ Clarify Brand Identity and Digital Presence
Similar on TelAve
- Melospeech Inc. Accepts Nomination for HealthTech Startup of the Year
- Verb™ Presents Features Vanguard Personalized Indexing: Utilizing Advanced Tax-Loss Harvesting Technology
- UK Financial Ltd Announces A Special Board Meeting Today At 4PM: Orders MCAT Lock on CATEX, Adopts ERC-3643 Standard, & Cancels $0.20 MCOIN for $1
- From Cheer to Courtroom: The Hidden Legal Risks in Your Holiday Eggnog
- Record Revenues, Debt-Free Momentum & Shareholder Dividend Ignite Investor Attention Ahead of 2026–2027 Growth Targets: IQSTEL (N A S D A Q: IQST)
- Cummings Graduate Institute for Behavioral Health Studies Celebrates New DBH Graduates
- $80M+ Backlog as Florida Statewide Contract, Federal Wins, and Strategic Alliance Fuel Next Phase of AI-Driven Cybersecurity Growth: Cycurion $CYCU
- High-Conviction CNS Disruptor Aiming to Transform Suicidal Depression, Ketamine Therapeutics, and TMS - Reaching Millions by 2030
- Talagat Business Academy Announces Joint Certificate Program With The University of Chicago Booth School of Business
- Slotozilla Launches New Report on How AI Is Reshaping Careers and Society
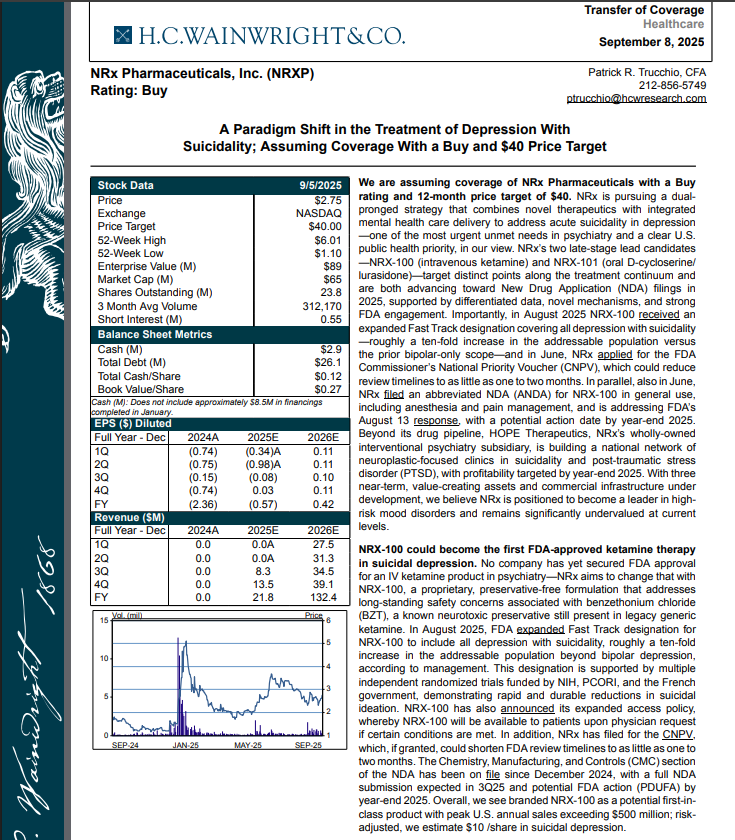
FDA Approval of Suitability Petition on Preservative-Free Ketamine Drug Supports $40 Analyst Target; $3 Billion Suicidal Depression Market: $NRXP
TelAve News/10876545
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Suitability Petition is required for shift from multidose packaging of ketamine to single-patient dose preservative free ketamine
MIAMI - TelAve -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
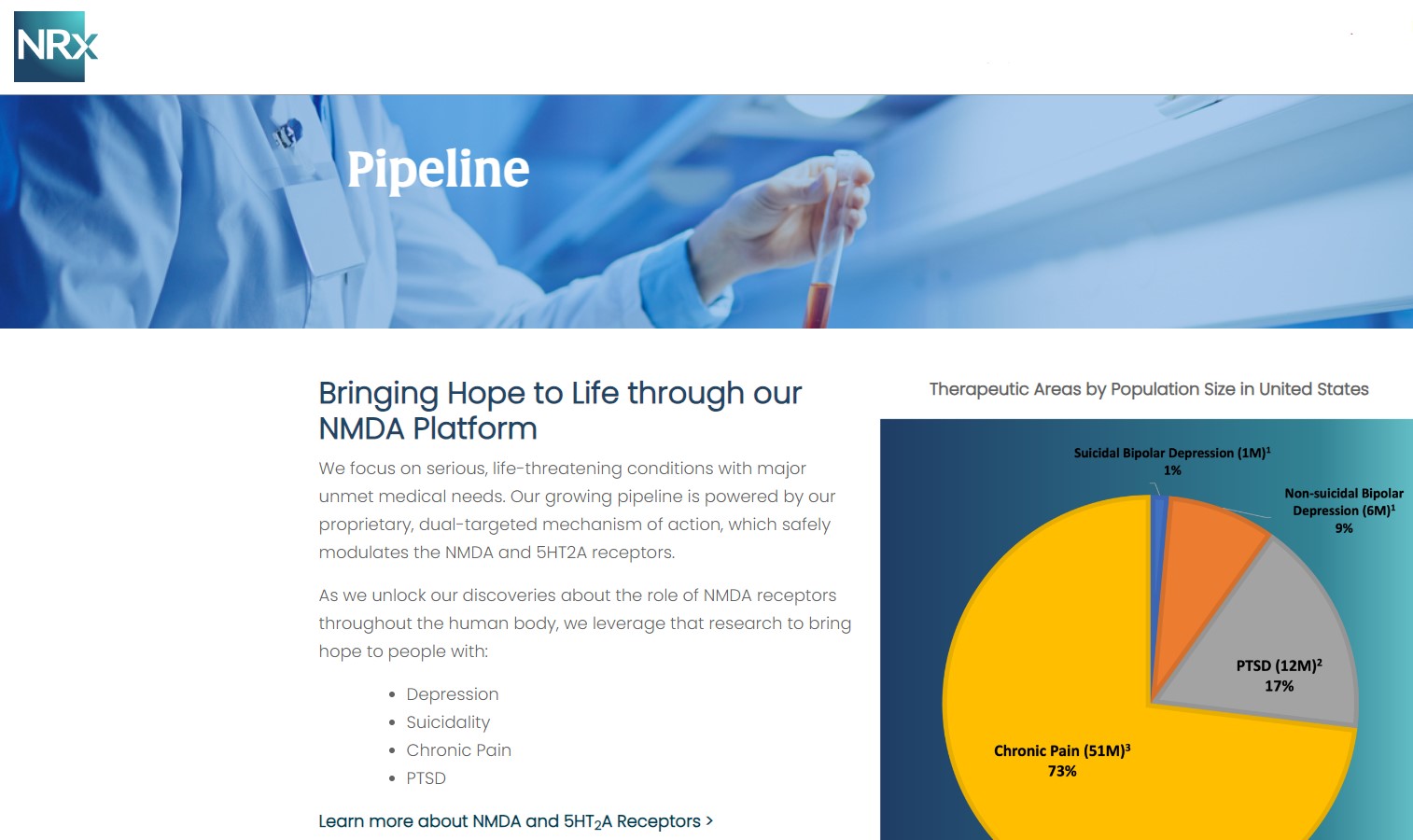
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on TelAve News
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine
On September 24th NRXP announced that it was notified by the United States Food and Drug Administration (FDA) that a Suitability Petition has been granted for the strength proposed by the Company for its planned single-patient, preservative-free ketamine product (KETAFREE™).
Currently, ketamine is sold in multi-dose vials that contain Benzethonium Chloride, a toxic preservative. The Suitability Petition that has been granted enables immediate re-filing of the NRXP Abbreviated New Drug Application for KETAFREE™. NRXP believes that this proposed product addresses two critical policy objectives as articulated by the current administration: (1) the re-shoring of strategically important drugs, particularly sterile products from foreign manufacturing sources, and (2) the "Make America Healthy Again" (MAHA) objective of removing toxic preservatives and colorants from foods and drugs. These objectives have been articulated on numerous occasions by FDA and HHS leadership.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents. NRXP believes that its current cash position will support operations into 2026 and provide sufficient capital to reach expected regulatory inflection points.
More on TelAve News
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on TelAve News
- Verb™ Presents Features Vanguard Personalized Indexing: Utilizing Advanced Tax-Loss Harvesting Technology
- UK Financial Ltd Announces A Special Board Meeting Today At 4PM: Orders MCAT Lock on CATEX, Adopts ERC-3643 Standard, & Cancels $0.20 MCOIN for $1
- 6 Holiday Looks That Scream "Old Money" But Cost Less Than Your Christmas Tree
- From Cheer to Courtroom: The Hidden Legal Risks in Your Holiday Eggnog
- Controversial Vegan Turns Rapper Launches First Song, "Psychopathic Tendencies."
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine
On September 24th NRXP announced that it was notified by the United States Food and Drug Administration (FDA) that a Suitability Petition has been granted for the strength proposed by the Company for its planned single-patient, preservative-free ketamine product (KETAFREE™).
Currently, ketamine is sold in multi-dose vials that contain Benzethonium Chloride, a toxic preservative. The Suitability Petition that has been granted enables immediate re-filing of the NRXP Abbreviated New Drug Application for KETAFREE™. NRXP believes that this proposed product addresses two critical policy objectives as articulated by the current administration: (1) the re-shoring of strategically important drugs, particularly sterile products from foreign manufacturing sources, and (2) the "Make America Healthy Again" (MAHA) objective of removing toxic preservatives and colorants from foods and drugs. These objectives have been articulated on numerous occasions by FDA and HHS leadership.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents. NRXP believes that its current cash position will support operations into 2026 and provide sufficient capital to reach expected regulatory inflection points.
More on TelAve News
- Inside the Fight for Affordable Housing: Avery Headley Joins Terran Lamp for a Candid Bronx Leadership Conversation
- Canterbury Hotel Group Announces the Opening of the TownePlace Suites by Marriott Portland Airport
- Heritage at South Brunswick's Resort-Style Amenities for Any Age and Every Lifestyle
- T-TECH Partners with Japan USA Precision Tools for 2026 US Market Development of the New T-TECH 5-Axis QUICK MILL™
- Record Revenues, Debt-Free Momentum & Shareholder Dividend Ignite Investor Attention Ahead of 2026–2027 Growth Targets: IQSTEL (N A S D A Q: IQST)
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Stocks
0 Comments
Latest on TelAve News
- Explosive Growth in U.S. Cryptocurrency Cloud Mining Sets The Stage for New Platform Launch with Daily Rewards in a Transparent Revenue-Share Model
- Qtex Cierra Ronda de $7 Millones para Estandarizar la Banca Transfronteriza en los Mercados Emergentes de Latinoamérica
- America's Most Festive Garages Wanted for Garage.com's 2025 Holiday Contest
- FDA Accepts ANDA for KETAFREE™ as Analyst Sets $34 Price Target for NRx Pharmaceuticals: (N A S D A Q : NRXP) NRx is Poised for a massive Breakthrough
- BEC Technologies Expands MX-220 5G Industrial Router Series for Edge Connectivity
- "Latino Leaders Speak: Personal Stories of Struggle and Triumph, Volume II" Documents the Truth About Latino Excellence and Impact on American Society
- Broadway Smile Boutique Unveils Modern Website for Enhanced Patient Experience
- Oklahoma and Starlink Local Installers getting it done!
- Fenix Consulting Group Expands Orange County Office to Meet Growing Client Demand
- Signature Smiles Dental Group Unveils New User-Friendly Website
- CCHR: New Data Shows Millions of U.S. Children Caught in Escalating Psychiatric Polypharmacy
- QwickContractReview.com Launches $19 Contract Review Service to Protect Consumers from Hidden Contract Risks
- 100% Bonus Depreciation Places New Spotlight on Off The Hook Yacht Sales Inc. (N Y S E: OTH) as a Major Player in the $57 Billion U.S. Marine Market
- CNCPW Benchmarks Global Industry Standards: Integrating SEC Compliance with 3 Million TPS Architecture for Institutional Infrastructure
- The Patina Collective & Artist Jesse Draxler Debut "The Machine of Loving Grace"
- Smile! Dental Center Named 2025 "Best Dentist" in North Pittsburgh, Celebrating High-Tech Care and Heartfelt Service
- Dr. Johnny Shanks, As Seen on TV, Announces 20% Off Dental Implant Treatments | Tennessee's Leading All-on-X Provider
- Star Sleep & Wellness Expands to Pearland, Texas — Bringing Life-Changing Sleep Care to More Communities
- Fort Lauderdale Dentist Dr. Taskonak & IN A DAY SMILE Receive Emmy Nomination for Life-Changing Documentary "The Weight of a Smile"
- Men's Health Network Highlights Major 2025 Achievements & Launches New Donation Platform For Greater Impact