Popular on TelAve
- Community Redevelopment Inc.Signs Gold Production Contract with Mine CA Gold Ltd., - 175
- CCHR: Why Psychiatric Detainment and Drugging Cannot Deliver Public Safety - 162
- Two Weeks Left: Secure Your Spot at the First OpenSSL Conference 2025 in Prague - 161
- NFL Yearbook Advertising Deal Signed Across 25 Stadiums for AI Powered Sports, Entertainment and Gaming Leader: SEGG Media $SEGG - 154
- Olga Torres Named to the 2025 Texas Super Lawyers List - 149
- Boston Industrial Solutions Introduces Natron® UVPX Series UV-LED Curing Screen Printing Inks - 148
- Powerful Points Illustrate Highly Undervalued Stock Price for Global Communications Leader: IQSTEL, Inc. (N A S D A Q: IQST) - 146
- DB Landscape Co. Brings Modern Outdoor Living to Coastal Communities - 146
- PlaceBased Expands NYC Presence with Strategic DOOH/OOH Asset Growth and Appoints OOH Industry Veteran Scott Nemeroff as EVP of National Accounts - 145
- Planetary Constitution Celebrates First Anniversary as Space Policy Shifts Toward Militarization - 141
Similar on TelAve
- Tami Goveia Enters FabOver40, Inspiring Hollywood Legacy for Breast Cancer Cause
- Swidget Launches Luminance™ to Help Schools Achieve Alyssa's Law Compliance
- MDRN MUSE Expands Insurance Network Coverage to Include Delta Dental & Cigna
- Physician Calls for States Nationwide to Ensure ADA Compliance in Independent Commissions
- MEDIA ADVISORY - Strengthening Children's Mental Health Across New Jersey
- NumberSquad Launches Year‑Round Tax Planning Package for Small Businesses and the Self‑Employed
- GlexScale launches a unified model for sustainable SaaS expansion across EMEA
- Zero-Trust Architecture: NJTRX Addresses 60% of U.S. Investors' Custody Security Concerns
- Sub-Millisecond Trading Platform: HNZLLQ Introduces Unified Gateway for Philippine Digital Asset Traders
- $2.1B Theft Losses: Bitquore Launches 1M+ TPS Platform with 95% Offline Asset Protection for U.S. Traders
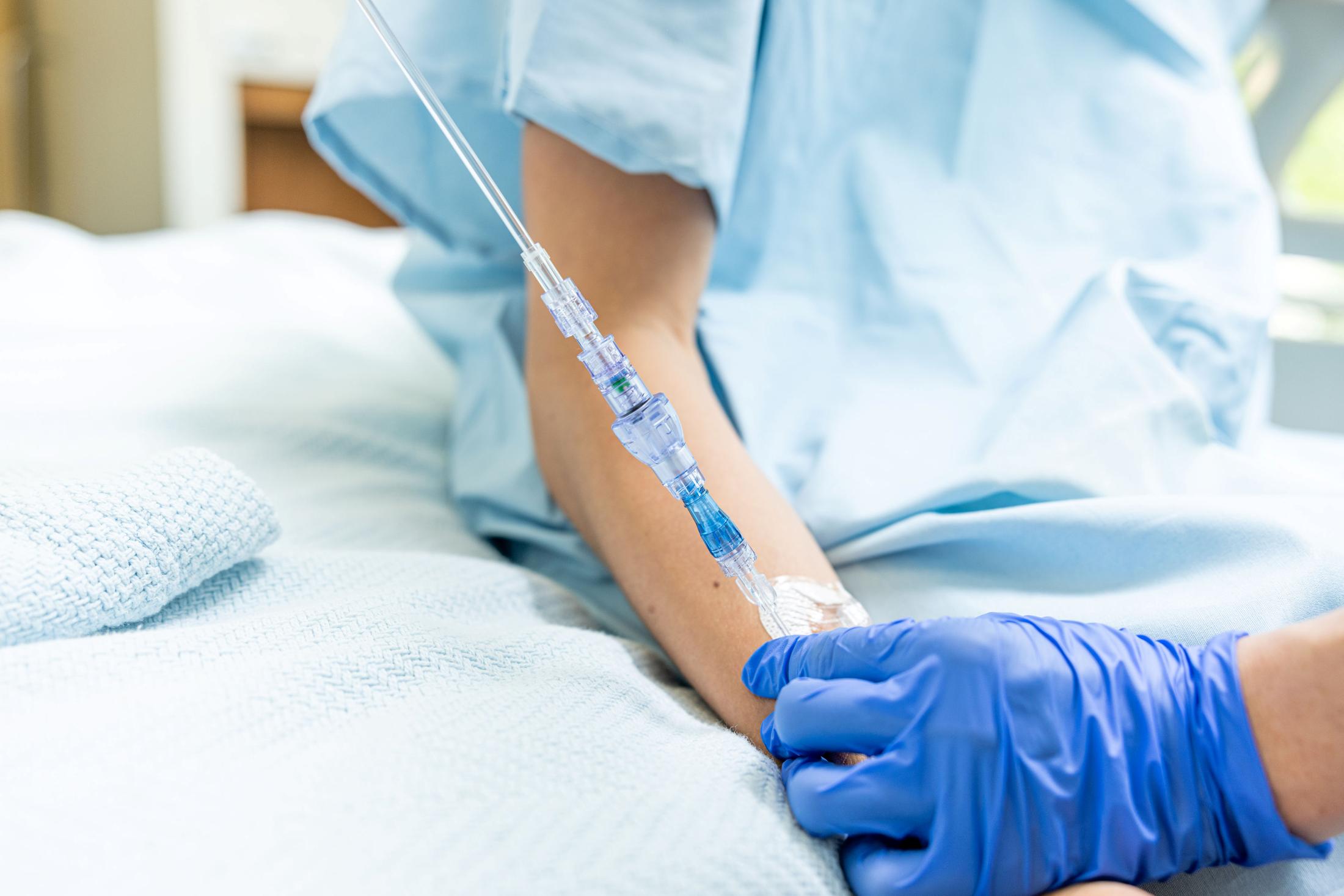
Lineus Medical Obtains CE Mark for Flagship Product SafeBreak Vascular
TelAve News/10877137
FAYETTEVILLE, Ark. - TelAve -- Lineus Medical has obtained CE Mark certification for its flagship product, SafeBreak® Vascular, the only breakaway device for IV lines clinically proven to reduce multiple IV complications.1 With this certification, SafeBreak Vascular is now cleared for use across the European Union. This approval expands SafeBreak Vascular's clearance from 5 countries to 32, spanning across more than three continents. Lineus Medical is actively partnering with distributors across Europe to expand hospital access to SafeBreak worldwide.
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on TelAve News
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on TelAve News
- Phinge Mobile Pop-Up Stores Previewing Its Patented Modular Earbuds & Magnetically Connectable Phones, Tablets & Gaming Systems, Coming to Your Town
- Physician Calls for States Nationwide to Ensure ADA Compliance in Independent Commissions
- MEDIA ADVISORY - Strengthening Children's Mental Health Across New Jersey
- Stop Paying Cloud Storage Fees: Make Money From Your Data That You Own, Through Netverse Verified App-less Platform & Hardware From Phinge
- NumberSquad Launches Year‑Round Tax Planning Package for Small Businesses and the Self‑Employed
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
Source: Lineus Medical
Filed Under: Technology
0 Comments
Latest on TelAve News
- $2.1B Theft Losses: Bitquore Launches 1M+ TPS Platform with 95% Offline Asset Protection for U.S. Traders
- America Anesthesia Partners Unveils New User-Friendly Website
- Hiclean Tools Releases HCX2100 Electric Pressure Washer
- Bùng Nổ Ra Mắt, AALIVE Tung Gói Thưởng 68% và Dàn Game Thuần Việt Hấp Dẫn
- ARCH Dental + Aesthetics Offers Free Consultations for New Patients
- Maisano Brothers Inc. Expands National Paving Division Into Tampa, Florida
- Multi-Signature Cold Storage: Keyanb Introduces Institutional-Grade Asset Protection for Chilean Crypto Traders
- NKSCX Introduces Zero-Knowledge Proof of Solvency for U.S. Traders Amid $6.5 Billion Fraud Crisis
- New Oasis International Foundation Announces Strategic Partnership Network Across 15 Countries to Advance Community-Led Economic Development
- Some Music for Donald's Bad Day
- New You Smile Dental Implant Center Expands Office
- $8 Billion High-Margin National Gentlemen's Club Market Targeted by Acquisition Strategy Incorporating the Successful Peppermint Hippo™ Brand: $TRWD
- Why Indian Game Development Companies Are Shaping the Future of Global Gaming
- Cold Storage and Proof-of-Reserves: BTXSGG Launches Institutional-Grade Asset Protection for Filipino Traders
- Redesigning the Digital World Around People, Not Algorithms: Netverse Verified, App-less Technologies, Platform and Hardware is Coming From Phinge
- Why FIRE Enthusiasts Are Buying Businesses Instead of Just Saving Their Way to Freedom
- All About bail Bonds Expands Presence to Serve Houston Families
- Thousands to Ride to L.A. Children's Hospital This Halloween Night
- Essential Living Support Opens First VA Medical Foster Home in Cheyenne, Wyoming
- Six-Figure Chicks Book Series 96 Authors, 6 Volumes Published to Empower and Mentor Women Nationwide