Popular on TelAve
- Report Outlines Key Questions for Individuals Exploring Anxiety Treatment Options in Toronto
- $1 Million Share Repurchase Signals Confidence as Off The Hook YS Scales a Tech-Driven Platform in the $57 Billion U.S. Marine Market
- Premium Bail Bonds Proudly Sponsors BOFAB BBQ Team at the 2026 Lakeland Pigfest
- Elizabeth McLaughlin, Founder and CEO of Red Wagon Group, named 2026 Presidential Leadership Scholar
- David Boland, Inc. Awarded $54.3M Construction Contract by U.S. Army Corps of Engineers, Savannah District
- Lacy Hendricks Earns Prestigious MPM® Designation from NARPM®
- CES Spotlight Highlights Need for Strategic Review as Throughput Demands Evolve
- Former Google Search Team Member Launches AI-Powered SEO Consultancy in Las Vegas
- Jones Sign Rebrands as Jones to Reflect Growth, Innovation, and Expanded Capabilities
- UK Financial Ltd Makes History as MayaCat (SMCAT) Becomes the World's First Exchange-Traded ERC-3643 Security Token
Similar on TelAve
- Does EMDR Really Work? New Article Explores How Trauma Gets Stuck in the Brain and How Healing Begins
- New Medium Article Explores Why Emotional Conversations Fail and What Most People Don't Understand About Connection
- $80 Million Revenue Backlog for AI Cybersecurity Company Building the Future of Integrated Cybersecurity and Public Safety: $CYCU
- Slick Cash Loan shares credit score tips for borrowers using bad credit loans
- Crossroads4Hope Welcomes New Trustees to Board of Directors as Organization Enters 25th Year of Caring
- UK Financial Ltd Advances Compliance Strategy With January 30th CATEX Exchange Listing Of Maya Preferred PRA Preferred Class Regulated Security Token
- Daniel Kaufman Launches a Vertically Integrated Real Estate and Investment Platform
- Impact Futures Group expands through acquisition of specialist healthcare sector training provider Caring for Care
- Powering the AI, Defense and Aerospace Future with Energy Infrastructure and Digital Asset Strength: KULR Technology Group, Inc. $KULR
- $10 Price Target in Think Equity Report Supported by Inventory Financing Floorplan Boot to $60 Million for 2026 Sales Growth in Pre-Owned Boats: $OTH
$750 Million Market on Track to $3.35 Billion by 2034: $NRXP Launches First-in-Florida "One Day" Depression Treatment in Partnership with Ampa Health
TelAve News/10880867
Analyst D. Boral Targets NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP at $34 Per Share — Pioneering Breakthroughs in Treatment-Resistant Depression and Chronic Pain
MIAMI - TelAve -- NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP), a clinical-stage biopharmaceutical innovator developing breakthrough therapeutics for central nervous system disorders, has emerged as a potential leader in the next generation of psychiatric care. With the global ketamine market projected to expand from $750 million to $3.35 billion by 2034, NRXP is strategically positioned to capture a substantial share through a suite of FDA-designated and investigational treatments aimed at addressing urgent mental health needs.
Revolutionary "ONE-D" Depression Treatment Launches in Florida
NRx recently announced the first-in-Florida launch of its "One Day" (ONE-D) depression treatment, developed in partnership with Ampa Health. The ONE-D protocol uses Ampa's advanced Transcranial Magnetic Stimulation (TMS) device — the first reported technology capable of achieving remission from treatment-resistant depression in a single day.
Unlike conventional 90-day TMS regimens, the ONE-D treatment integrates a single day of precision TMS with D-cycloserine and lisdexamfetamine (both utilized under physician supervision), achieving up to 87% response and 72% remission rates in peer-reviewed nonrandomized studies.
The technology is initially deployed at multiple NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, with expansion to six Florida locations by year-end 2025 under the direction of Dr. Rebecca Cohen, Medical Director of HOPE Clinics.
More on TelAve News
NRX-101: A Breakthrough Therapy for Suicidal Bipolar Depression
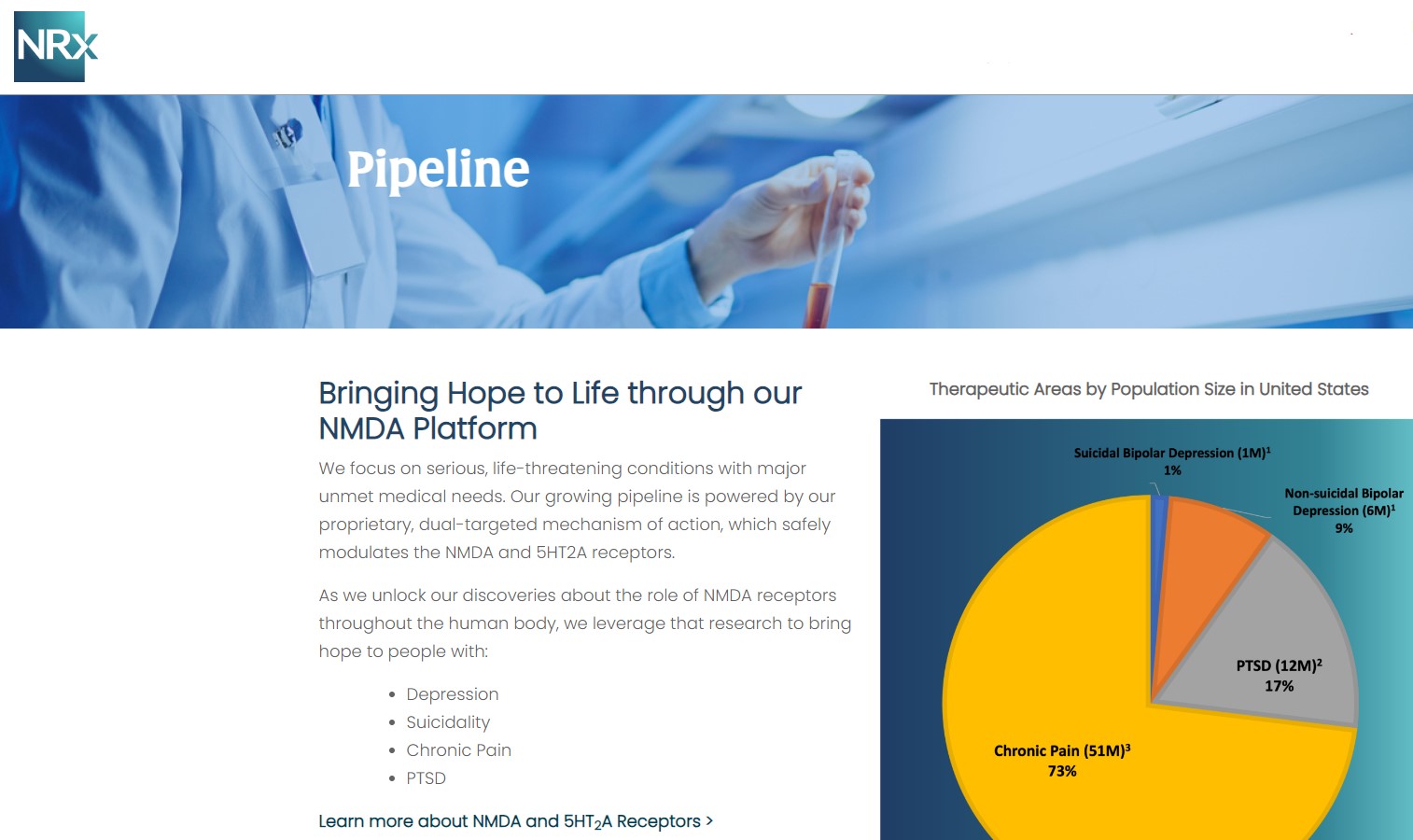
Central to NRx's pipeline is NRX-101, an FDA-designated Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. The therapy leverages the company's proprietary NMDA platform and is being developed in partnership with Alvogen Pharmaceuticals.
NRX-101 also holds potential as a non-opioid pain treatment and for other indications such as complicated urinary tract infections (UTIs). With over 13 million Americans seriously considering suicide each year (CDC), NRXP's drug candidates could address one of the nation's most pressing health crises.
KETAFREE™: FDA-Focused Push to Modernize Ketamine Treatment
NRXP recently re-filed its Abbreviated New Drug Application (ANDA) for KETAFREE™, a preservative-free IV ketamine formulation, after securing FDA approval of its Suitability Petition. By eliminating benzethonium chloride (BZT) — a preservative linked to neurotoxicity — KETAFREE™ represents a safer, next-generation alternative to traditional ketamine products.
The company has also petitioned the FDA to formally remove BZT from IV formulations, citing expert toxicological data and the growing demand for preservative-free therapies in modern medicine. Given the ongoing ketamine shortage reported by the American Society of Hospital Pharmacists, NRXP's KETAFREE™ could fill a critical supply gap and strengthen its market positioning.
Strategic Acquisitions and Growth Initiatives
NRXP recently completed the acquisition of Dura Medical, an EBITDA-positive network of interventional psychiatry clinics delivering advanced therapies for depression and PTSD. This acquisition, along with pending deals for Neurospa TMS and Cohen & Associates, expands NRXP's reach to more than eight Florida clinic locations, reinforcing its vertically integrated mental health care model.
More on TelAve News
To accelerate this rollout, NRXP secured $7.8 million in debt financing from Universal Capital, LLC to fund further HOPE Clinic acquisitions.
Additionally, NRXP has accepted non-binding licensing terms for NRX-100, a drug candidate expected to generate over $300 million in potential milestone payments and tiered double-digit royalties upon commercialization.
Strong Analyst Endorsement
In a recently released report, D. Boral Research issued a Buy rating on NRXP with a $34 price target, citing the company's expanding clinical pipeline, growing footprint in the interventional psychiatry sector, and first-mover advantage in the transformative ONE-D treatment platform.
A Vision for Transforming Mental Health
From its FDA Fast Track designation for IV ketamine (NRX-100) to its breakthrough therapy designation for NRX-101, NRx Pharmaceuticals continues to redefine the boundaries of psychiatric medicine. Its commitment to science-driven innovation — now coupled with real-world clinic deployment through HOPE Clinics — positions NRXP as both a therapeutic pioneer and a potentially lucrative opportunity in the rapidly expanding mental health market.
Company Contact:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
📍 Wilmington, DE
Matthew Duffy, Chief Business Officer
📧 mduffy@nrxpharma.com | ☎️ (484) 254-6134
🌐 www.nrxpharma.com
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Revolutionary "ONE-D" Depression Treatment Launches in Florida
NRx recently announced the first-in-Florida launch of its "One Day" (ONE-D) depression treatment, developed in partnership with Ampa Health. The ONE-D protocol uses Ampa's advanced Transcranial Magnetic Stimulation (TMS) device — the first reported technology capable of achieving remission from treatment-resistant depression in a single day.
Unlike conventional 90-day TMS regimens, the ONE-D treatment integrates a single day of precision TMS with D-cycloserine and lisdexamfetamine (both utilized under physician supervision), achieving up to 87% response and 72% remission rates in peer-reviewed nonrandomized studies.
The technology is initially deployed at multiple NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, with expansion to six Florida locations by year-end 2025 under the direction of Dr. Rebecca Cohen, Medical Director of HOPE Clinics.
More on TelAve News
- Crossroads4Hope Welcomes New Trustees to Board of Directors as Organization Enters 25th Year of Caring
- PromptBuilder.cc Launches AI Prompt Generator Optimized For ChatGPT, Gemini, Grok & Claude
- UK Financial Ltd Advances Compliance Strategy With January 30th CATEX Exchange Listing Of Maya Preferred PRA Preferred Class Regulated Security Token
- NOW OPEN - New Single Family Home Community in Manalapan
- Kintetsu And Oversee Announce New Partnership
NRX-101: A Breakthrough Therapy for Suicidal Bipolar Depression
Central to NRx's pipeline is NRX-101, an FDA-designated Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. The therapy leverages the company's proprietary NMDA platform and is being developed in partnership with Alvogen Pharmaceuticals.
NRX-101 also holds potential as a non-opioid pain treatment and for other indications such as complicated urinary tract infections (UTIs). With over 13 million Americans seriously considering suicide each year (CDC), NRXP's drug candidates could address one of the nation's most pressing health crises.
KETAFREE™: FDA-Focused Push to Modernize Ketamine Treatment
NRXP recently re-filed its Abbreviated New Drug Application (ANDA) for KETAFREE™, a preservative-free IV ketamine formulation, after securing FDA approval of its Suitability Petition. By eliminating benzethonium chloride (BZT) — a preservative linked to neurotoxicity — KETAFREE™ represents a safer, next-generation alternative to traditional ketamine products.
The company has also petitioned the FDA to formally remove BZT from IV formulations, citing expert toxicological data and the growing demand for preservative-free therapies in modern medicine. Given the ongoing ketamine shortage reported by the American Society of Hospital Pharmacists, NRXP's KETAFREE™ could fill a critical supply gap and strengthen its market positioning.
Strategic Acquisitions and Growth Initiatives
NRXP recently completed the acquisition of Dura Medical, an EBITDA-positive network of interventional psychiatry clinics delivering advanced therapies for depression and PTSD. This acquisition, along with pending deals for Neurospa TMS and Cohen & Associates, expands NRXP's reach to more than eight Florida clinic locations, reinforcing its vertically integrated mental health care model.
More on TelAve News
- TMI Apparel Drops the "I'm Not Crazy, I'm Curated" Crop Hoodie
- Save 10 Percent Off KeysCaribbean's Newly Added Luxury Vacation Home in Marathon
- Why 'Instant-Liquidity' Gaming is Dominating the Nordic Tech Demographic
- STATEMENT: Shincheonji on Religious Freedom Controversy
- SheRising: Friends in Solidarity Hosts Webinar on Women in South Sudan
To accelerate this rollout, NRXP secured $7.8 million in debt financing from Universal Capital, LLC to fund further HOPE Clinic acquisitions.
Additionally, NRXP has accepted non-binding licensing terms for NRX-100, a drug candidate expected to generate over $300 million in potential milestone payments and tiered double-digit royalties upon commercialization.
Strong Analyst Endorsement
In a recently released report, D. Boral Research issued a Buy rating on NRXP with a $34 price target, citing the company's expanding clinical pipeline, growing footprint in the interventional psychiatry sector, and first-mover advantage in the transformative ONE-D treatment platform.
A Vision for Transforming Mental Health
From its FDA Fast Track designation for IV ketamine (NRX-100) to its breakthrough therapy designation for NRX-101, NRx Pharmaceuticals continues to redefine the boundaries of psychiatric medicine. Its commitment to science-driven innovation — now coupled with real-world clinic deployment through HOPE Clinics — positions NRXP as both a therapeutic pioneer and a potentially lucrative opportunity in the rapidly expanding mental health market.
Company Contact:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
📍 Wilmington, DE
Matthew Duffy, Chief Business Officer
📧 mduffy@nrxpharma.com | ☎️ (484) 254-6134
🌐 www.nrxpharma.com
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on TelAve News
- OpenSSL Corporation Opens 2026 Advisory Committees' Elections: Shape the Future!
- Steve Everett Jr. Named President of L.T. Hampel Corporation
- Acuvance Acquires ROI Healthcare Solutions, Building a Dedicated Healthcare ERP Practice
- Max Tucci Award-Winning Media Powerhouse Launches New Podcast —Executive Produced by Emmy-Winning Daytime Icons Suzanne Bass & Fran Brescia Coniglio
- MILBERT.ai Brings Real Time Session Defense to Google Workspace and Google Cloud
- Appliance Outlet Caps Off a Record-Setting 2025 Nationwide, Gears Up for Even Greater Growth in 2026
- Home Prices Just Hit 5X Median Income — So Americans Are Buying Businesses Instead of Houses
- CCHR White Paper Urges Government Crackdown on Troubled Teen and For-Profit Psychiatric Facilities
- Still Searching for the Perfect Valentine's Gift? Lick Personal Oils Offers Romantic, Experience-Driven Alternatives to Traditional Presents
- Boston Industrial Solutions' BPA Certified BX Series Raises the Bar for Pad Printing Inks
- Boston Corporate Coach™ Sets Global Standard for Executive Chauffeur Services Across 680 Cities
- UK Financial Ltd Announces CoinMarketCap Supply Verification And Market Positioning Review For Regulated Security Tokens SMPRA And SMCAT
- Sharpe Automotive Redefines Local Car Care with "Transparency-First" Service Model in Santee
- Secondesk Launches Powerful AI Tutor That Speaks 20+ Languages
- Automation, innovation in healthcare processes featured at international conference in Atlanta
- A High-Velocity Growth Story Emerges in Marine and Luxury Markets
- $26 Billion Global Market by 2035 for Digital Assets Opens Major Potential for Currency Tech Company with ATM Expansion and Deployment Plans Underway
- Peernovation 365 is Now Available
- Snap-a-Box Brings Texas' First Robot-Cooked Chinese Takeout to Katy–Fulshear
- UK Financial Ltd Makes History as MayaCat (SMCAT) Becomes the World's First Exchange-Traded ERC-3643 Security Token