Popular on TelAve
- Phinge CEO Ranked #1 Globally by Crunchbase for the Last Week, Will Be in Las Vegas Jan. 4-9, the Week of CES to Discuss Netverse & IPO Coming in 2026 - 137
- UK Financial Ltd Board of Directors Establishes Official News Distribution Framework and Issues Governance Decision on Official Telegram Channels
- Christy Sports donates $56K in new gear to SOS Outreach to help kids hit the slopes
- Creative Investment Research Warns AT&T Rollback Undermines Market Integrity
- UK Financial Ltd Launches U.S. Operations Following Delaware Approval
- Women's Everyday Safety Is Changing - The Blue Luna Shows How
- Microgaming Unveils Red Papaya: A New Studio Delivering Cutting-Edge, Feature-Rich Slots
- Nextvisit Co-Founder Ryan Yannelli Identifies Six Critical Factors for Behavioral Health Providers Evaluating AI Scribes in 2026
- Costa Oil - 10 Minute Oil Change Surpasses 70 Locations with Construction of San Antonio, TX Stores — Eyes Growth Via Acquisition or Being Acquired
- TimelyBill at ITEXPO 2026: Modern Billing for Modern Telecom
Similar on TelAve
- New Analysis Reveals Most Patients Discontinue Weight Loss Drugs Within First Year
- UK Financial Ltd Receives Recognition In Platinum Crypto Academy's "Cryptonaire Weekly"
- Preston Dermatology & Skin Surgery Center Wins Gold and Bronze in Prestigious Annual DIAMOND Awards
- $1 Million Share Repurchase Signals Confidence as Off The Hook YS Scales a Tech-Driven Platform in the $57 Billion U.S. Marine Market
- Trends Journal's Top Trends of 2026
- The Stork Foundation Announces 2025 Year-End Impact and Grant Awards Amid Rising National Demand
- Breakout Phase for Public Company: New Partnerships, Zero Debt, and $20 Million Growth Capital Position Company for 2026 Acceleration
- Japan's Patented "Hammock'n" Smartphone Band Targets Hand Fatigue From Long Phone Use
- CCHR: Harvard Review Exposes Institutional Corruption in Global Mental Health
- Global License Exclusive Secured for Emesyl OTC Nausea Relief, Expanding Multi-Product Growth Strategy for Caring Brands, Inc. (N A S D A Q: CABR)
$750 Million Market Set to Soar to $3.35 Billion by 2034 as Florida Launches First-in-Nation One-Day: NRx Pharmaceuticals (N A S D A Q: NRXP) $NRXP
TelAve News/10880825
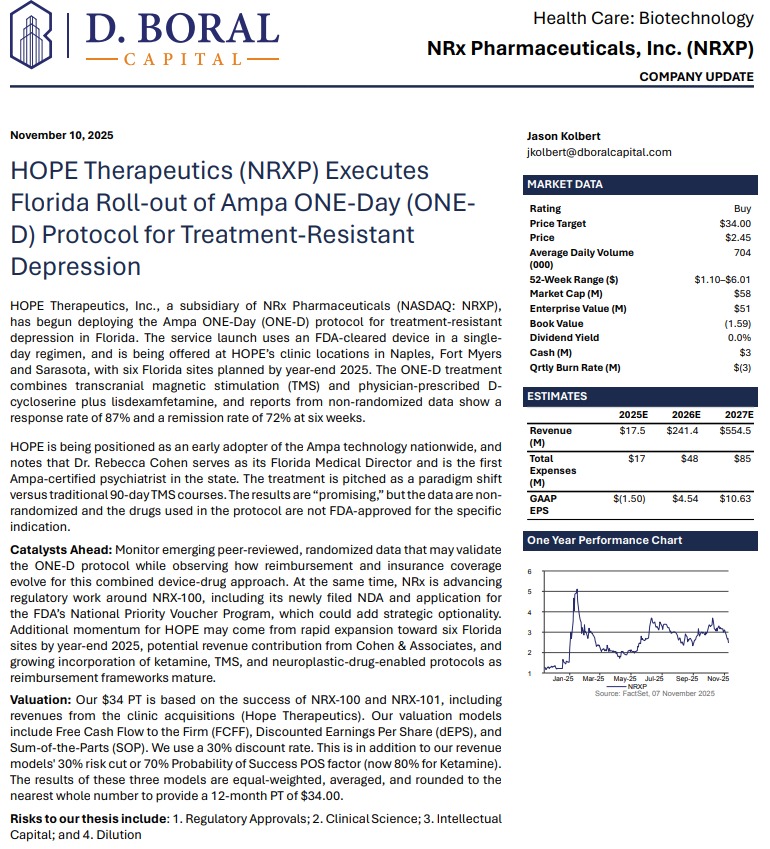
H.C. Wainwright Initiates Coverage with $34 Price Target, Citing Paradigm Shift in Depression Treatment. NRx Pharmaceuticals (N A S D A Q: NRXP) $NRXP
MIAMI - TelAve -- NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP), a clinical-stage biopharmaceutical company pioneering NMDA-based therapeutics for central nervous system disorders, is emerging as a potential leader in the next generation of depression and suicidality treatments. With its innovative ONE-D (One Day Depression) protocol now launched in Florida and a $40 price target set by H.C. Wainwright, NRXP appears positioned for transformational growth in a rapidly expanding $3.35 billion global market.
A Major Step Forward: ONE-D Depression Treatment Launches in Florida
NRx Pharmaceuticals has initiated the first-in-Florida rollout of the ONE-D (One Day) Depression Treatment, in partnership with Ampa Health. The innovative protocol has shown breakthrough potential in achieving remission from treatment-resistant depression within a single day—an unprecedented leap forward for millions suffering from chronic depression.
Unlike traditional transcranial magnetic stimulation (TMS) therapies requiring up to 90 sessions over three months, the ONE-D approach combines a single-day TMS treatment with physician-prescribed compounds, including D-cycloserine, an active component of NRx's investigational NRX-101, and lisdexamfetamine. Early clinical data show an 87% response rate and 72% remission rate in nonrandomized studies.
The Ampa device is now available across NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, Florida—with six total locations expected by the end of 2025. This initiative marks one of the first national deployments of the Ampa ONE-D protocol.
More on TelAve News
Breakthrough Therapeutic Portfolio: NRX-101 and KETAFREE™
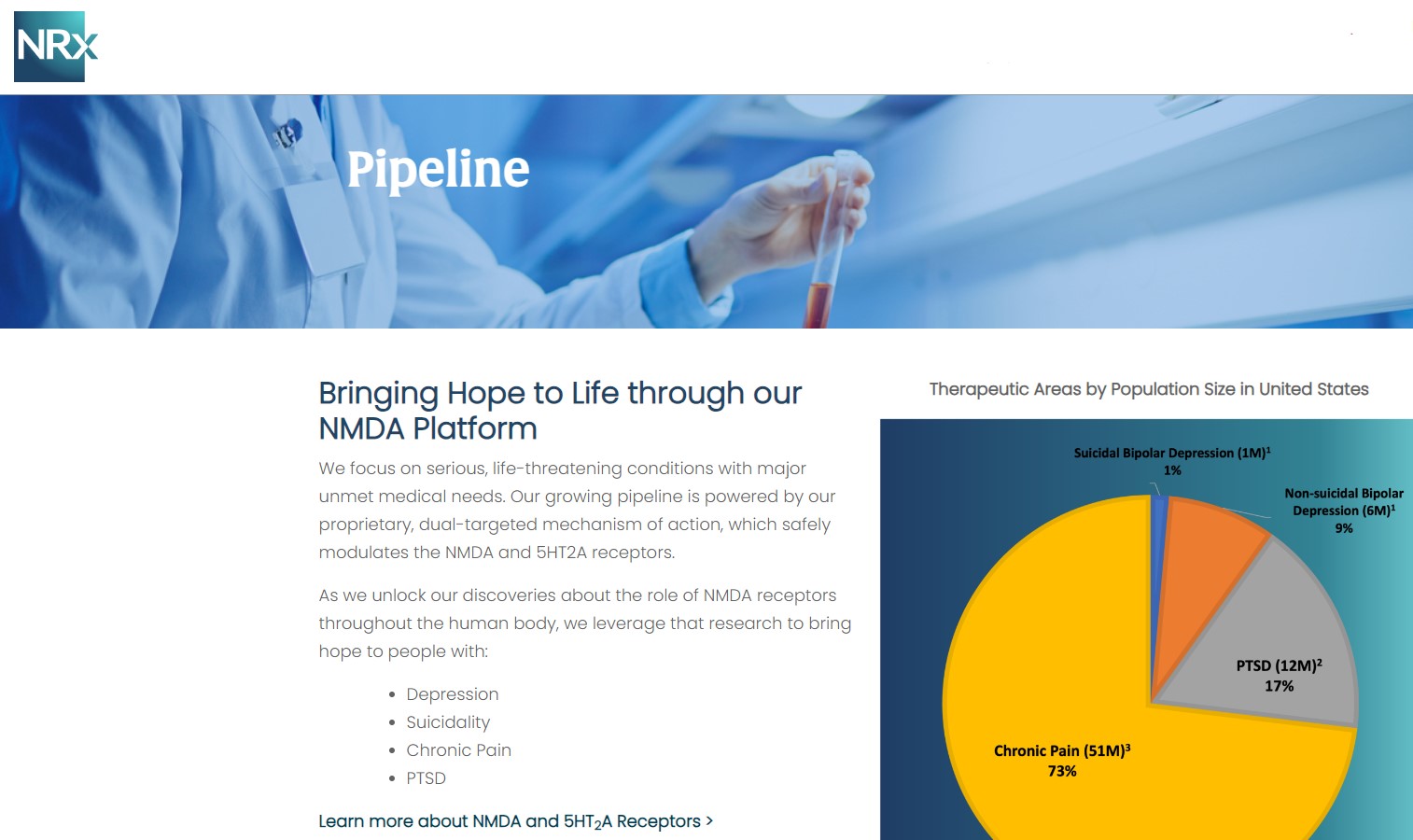
At the center of NRXP's drug pipeline is NRX-101, an FDA-designated Breakthrough Therapy targeting suicidal, treatment-resistant bipolar depression and chronic pain. The drug represents a potentially lifesaving option for the more than 13 million Americans who seriously consider suicide each year, according to the CDC.
NRXP is also advancing KETAFREE™, a preservative-free IV ketamine formulation, through a refiled Abbreviated New Drug Application (ANDA) with the FDA. This comes as the ketamine market—currently valued at $750 million—is projected to reach $3.35 billion globally by 2034.
Importantly, the FDA has granted approval of NRXP's Suitability Petition for its proposed strength of preservative-free ketamine, clearing the path for potential market entry amid ongoing national shortages of sterile ketamine formulations.
Strategic Expansion Through Dura Medical Acquisition
NRXP recently completed its acquisition of Dura Medical, a revenue-generating and EBITDA-positive network of precision psychiatry clinics along Florida's West Coast. The addition of Dura, alongside pending acquisitions such as Neurospa TMS and Cohen & Associates, positions NRXP to deliver an integrated continuum of mental health care spanning TMS, ketamine therapy, and advanced pharmacologic treatments for PTSD, depression, and chronic pain.
Robust Financial and Partnership Outlook
The company's growth strategy is supported by a $7.8 million debt financing deal with Universal Capital, LLC, aimed at expanding NRXP's HOPE Clinic network. In parallel, NRXP has accepted non-binding potential licensing terms for NRX-100, with the agreement representing over $300 million in milestone payments and tiered double-digit royalties—a major value inflection opportunity for investors.
More on TelAve News
Moreover, NRXP's partnership with Alvogen Pharmaceuticals supports the ongoing development and commercial readiness of NRX-101, further validating the company's clinical and commercial trajectory.
Analyst Confidence: H.C. Wainwright Issues "Buy" Rating and $40 Price Target
A recent analyst report from H.C. Wainwright & Co. titled "A Paradigm Shift in the Treatment of Depression with Suicidality" initiated coverage on NRx Pharmaceuticals with a Buy rating and a $34 price target. The report underscores NRXP's leadership in transforming how depression and suicidality are treated, leveraging FDA Fast Track and Breakthrough Therapy designations to accelerate commercialization.
"We view NRXP's approach as a paradigm shift in the treatment of depression with suicidality," wrote H.C. Wainwright analysts. "With novel drug candidates, a rapidly expanding clinic network, and strategic licensing opportunities, NRXP is uniquely positioned to capture substantial market share."
About NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
NRx Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company developing breakthrough therapeutics based on its NMDA receptor modulation platform for the treatment of central nervous system disorders, including suicidal bipolar depression, chronic pain, and PTSD.
Through its partnerships, innovative clinical pipeline, and integrated treatment model, NRXP is setting new standards in precision psychiatry and interventional mental health care.
For More Information:
Company: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com
Website: www.nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
A Major Step Forward: ONE-D Depression Treatment Launches in Florida
NRx Pharmaceuticals has initiated the first-in-Florida rollout of the ONE-D (One Day) Depression Treatment, in partnership with Ampa Health. The innovative protocol has shown breakthrough potential in achieving remission from treatment-resistant depression within a single day—an unprecedented leap forward for millions suffering from chronic depression.
Unlike traditional transcranial magnetic stimulation (TMS) therapies requiring up to 90 sessions over three months, the ONE-D approach combines a single-day TMS treatment with physician-prescribed compounds, including D-cycloserine, an active component of NRx's investigational NRX-101, and lisdexamfetamine. Early clinical data show an 87% response rate and 72% remission rate in nonrandomized studies.
The Ampa device is now available across NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, Florida—with six total locations expected by the end of 2025. This initiative marks one of the first national deployments of the Ampa ONE-D protocol.
More on TelAve News
- Preston Dermatology & Skin Surgery Center Wins Gold and Bronze in Prestigious Annual DIAMOND Awards
- David Boland, Inc. Awarded $54.3M Construction Contract by U.S. Army Corps of Engineers, Savannah District
- "Phinge Unveil™" Coming to Las Vegas to Showcase Netverse Patented Verified App-less Platform, AI & Modular Hardware Including Developer Conferences
- Elizabeth McLaughlin, Founder and CEO of Red Wagon Group, named 2026 Presidential Leadership Scholar
- U.S. Congressional Candidate Peter Coe Verbica on America's Asymmetric Crisis
Breakthrough Therapeutic Portfolio: NRX-101 and KETAFREE™
At the center of NRXP's drug pipeline is NRX-101, an FDA-designated Breakthrough Therapy targeting suicidal, treatment-resistant bipolar depression and chronic pain. The drug represents a potentially lifesaving option for the more than 13 million Americans who seriously consider suicide each year, according to the CDC.
NRXP is also advancing KETAFREE™, a preservative-free IV ketamine formulation, through a refiled Abbreviated New Drug Application (ANDA) with the FDA. This comes as the ketamine market—currently valued at $750 million—is projected to reach $3.35 billion globally by 2034.
Importantly, the FDA has granted approval of NRXP's Suitability Petition for its proposed strength of preservative-free ketamine, clearing the path for potential market entry amid ongoing national shortages of sterile ketamine formulations.
Strategic Expansion Through Dura Medical Acquisition
NRXP recently completed its acquisition of Dura Medical, a revenue-generating and EBITDA-positive network of precision psychiatry clinics along Florida's West Coast. The addition of Dura, alongside pending acquisitions such as Neurospa TMS and Cohen & Associates, positions NRXP to deliver an integrated continuum of mental health care spanning TMS, ketamine therapy, and advanced pharmacologic treatments for PTSD, depression, and chronic pain.
Robust Financial and Partnership Outlook
The company's growth strategy is supported by a $7.8 million debt financing deal with Universal Capital, LLC, aimed at expanding NRXP's HOPE Clinic network. In parallel, NRXP has accepted non-binding potential licensing terms for NRX-100, with the agreement representing over $300 million in milestone payments and tiered double-digit royalties—a major value inflection opportunity for investors.
More on TelAve News
- Jones Sign Rebrands as Jones to Reflect Growth, Innovation, and Expanded Capabilities
- $1 Million Share Repurchase Signals Confidence as Off The Hook YS Scales a Tech-Driven Platform in the $57 Billion U.S. Marine Market
- ELEOLUXE Sets Out a New Framework for Residential Renovation Intelligence
- Trends Journal's Top Trends of 2026
- CollabWait to Launch Innovative Waitlist Management Platform for Behavioral Health Services
Moreover, NRXP's partnership with Alvogen Pharmaceuticals supports the ongoing development and commercial readiness of NRX-101, further validating the company's clinical and commercial trajectory.
Analyst Confidence: H.C. Wainwright Issues "Buy" Rating and $40 Price Target
A recent analyst report from H.C. Wainwright & Co. titled "A Paradigm Shift in the Treatment of Depression with Suicidality" initiated coverage on NRx Pharmaceuticals with a Buy rating and a $34 price target. The report underscores NRXP's leadership in transforming how depression and suicidality are treated, leveraging FDA Fast Track and Breakthrough Therapy designations to accelerate commercialization.
"We view NRXP's approach as a paradigm shift in the treatment of depression with suicidality," wrote H.C. Wainwright analysts. "With novel drug candidates, a rapidly expanding clinic network, and strategic licensing opportunities, NRXP is uniquely positioned to capture substantial market share."
About NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
NRx Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company developing breakthrough therapeutics based on its NMDA receptor modulation platform for the treatment of central nervous system disorders, including suicidal bipolar depression, chronic pain, and PTSD.
Through its partnerships, innovative clinical pipeline, and integrated treatment model, NRXP is setting new standards in precision psychiatry and interventional mental health care.
For More Information:
Company: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com
Website: www.nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on TelAve News
- Breakout Phase for Public Company: New Partnerships, Zero Debt, and $20 Million Growth Capital Position Company for 2026 Acceleration
- Japan's Patented "Hammock'n" Smartphone Band Targets Hand Fatigue From Long Phone Use
- Reditus Group Introduces A New Empirical Model for Early-Stage B2B Growth
- CCHR: Harvard Review Exposes Institutional Corruption in Global Mental Health
- New Book: Customer loyalty is dead – telcos have been chasing a ghost
- Goatimus Launches Dynamic Context: AI Prompt Engineering Gets Smarter
- Global License Exclusive Secured for Emesyl OTC Nausea Relief, Expanding Multi-Product Growth Strategy for Caring Brands, Inc. (N A S D A Q: CABR)
- RNHA Affirms Support for President Trump as Nation Marks Historic Victory for Freedom
- American Laser Study Club Announces 2026 Kumar Patel Prize in Laser Surgery Recipients: Ann Bynum, DDS, and Boaz Man, DVM
- Lineus Medical Completes UK Registration for SafeBreak® Vascular
- Canyons & Chefs Announces Revamped Homepage
- $140 to $145 Million in 2026 Projected and Profiled in New BD Deep Research Report on its Position in $57 Billion US Marine Industry; N Y S E: OTH
- Really Cool Music Releases Its Fourth Single - "So Many Lost Years"
- MGN Logistics Acquires Fast Service LLC, Fueling MyMGN Marketplace Expansion and Supercharging Expedited Coverage Nationwide
- The Wait is Over: Salida Wine Festival Announces Triumphant 2026 Return After Seven-Year Hiatus
- Graduates With $40K in Student Debt Are Buying Businesses Instead of Taking Entry-Level Jobs
- Anne Seidman: Within the Lines
- How Democrats Made Healthcare More Expensive in 2026
- Inkdnylon Launches Bilingual Ask Inkdnylon Platform