Popular on TelAve
- Althea Gibson Honored as Final Release in U.S. Mint's American Women Quarters Program - 173
- Cyntexa Announces Updates to ChargeOn on Salesforce AppExchange
- Oklahoma and Starlink Local Installers getting it done! / now offering digital menu board installs
- Starlink Local Installers working with state of Minnesota (now offering digital menu board installs)
- Kudosity appoints Jules Holden to drive channel growth and expand offering in ecommerce and retail
- Own 327 Acres of American Prime Real Estate with 2 Miles Waterfront Worth In Millions for Just $7 — Worldwide Raffle Launched
- Nebraska and Starlink Local Installers working together for reliable internet
- Starlink Local Installers helping Wisconsin stay wired (now offering digital menu board installs)
- Dr. Alexander Eastman Returns to Suburban Hospital to Deliver Keynote on Crisis Leadership
- Introducing TimelyTAXES™: A High-Speed, In-House Tax Engine Built for Telecom
Similar on TelAve
- HBZBZL Unveils "Intelligent Ecosystem" Strategy: Integrating AI Analytics with Web3 Incubation
- Kaltra Launches Next-Gen MCHEdesign With Full Integration Into MCHEselect — Instant Simulation & Seamless Microchannel Coil Workflow
- A Well-Fed World, Youth Climate Save and PAN International Launch PHRESH: A Global Directory of Plant-Based Hunger Relief Organizations
- Trump's Executive Order Rescheduling Cannabis: Accelerating M&A in a Multibillion-Dollar Industry
- Documentary "Prescription for Violence: Psychiatry's Deadly Side Effects" Premieres, Exposes Link Between Psychiatric Drugs and Acts of Mass Violence
- Nextvisit Co-Founder Ryan Yannelli Identifies Six Critical Factors for Behavioral Health Providers Evaluating AI Scribes in 2026
- CredHub and Real Property Management Join Forces to Empower Franchise Owners with Rental Payment Credit Reporting Solutions
- Renowned Alternative Medicine Specialist Dr. Sebi and His African Bio Mineral Balance Therapy Are the Focus of New Book
- Psychiatric Drug Damage Ignored for Decades; CCHR Demands Federal Action
- Why Millions Are Losing Sexual Sensation, And Why It's Not Age, Hormones, or Desire
Acquisition of Kadima Neuropsychiatry Institute for CNS & Psychedelic Research Planned; New Drug Application for Suicidal Depression Treatment: $NRXP
TelAve News/10850235
NRx Pharmaceuticals, Inc.: Stock Symbol: NRXP IS Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
MIAMI - TelAve -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
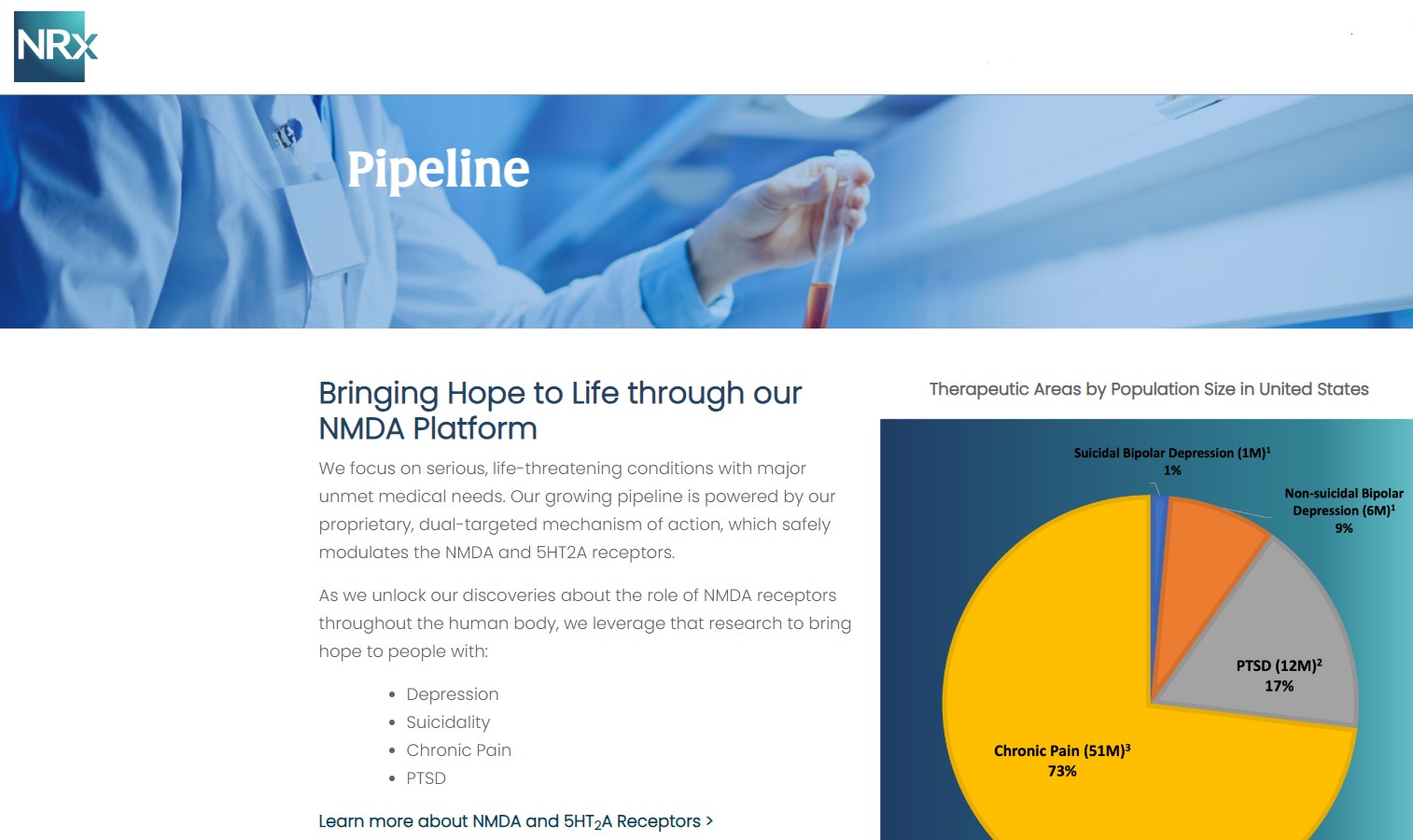
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Kadima Neuropsychiatry Institute Targeted as First Acquisition for HOPE Subsidiary International Network of Interventional Psychiatry Clinics.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research.
Completion of NDA Filing Expected in First Quarter of 2025
Plans to Participate in J.P. Morgan Healthcare Conference on January 13-16, 2025, in San Francisco, CA.
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. $NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
Kadima Neuropsychiatry Institute Expected First Acquisition for HOPE Subsidiary International Network of Interventional Psychiatry Clinics
More on TelAve News
On January 2nd NRXP announced the planned acquisition of the Kadima Neuropsychiatry Institute of La Jolla, CA, per the previously announced Letter of Intent, for the Company's HOPE subsidiary network. Kadima is expected to serve as the flagship clinic for HOPE's planned international network of interventional psychiatry clinics, designed to provide advanced treatments for debilitating diseases such as depression, anxiety and PTSD.
Kadima is one of the world's premier interventional psychiatry clinics and was among the first to introduce Ketamine Therapy for Central Nervous System (CNS) disorders at scale in the clinic setting. The clinic offers a full range of cutting-edge treatments for suicidal depression, anxiety, Post Traumatic Stress Disorder (PTSD) and other CNS disorders. Those treatment options include Ketamine Therapy, Spravato® (nasal esketamine), Transcranial Magnetic Stimulation as well as medication management.
Kadima also has a robust research division and is a leading investigative site for innovative CNS treatments, specializing in psychedelic research, for which it has served as a leading site in nearly all major clinical trials in this area. Kadima has contracts in place with the US Department of Veterans Affairs and also treats active-duty military personnel in the US Department of Defense under Tricare and other treatment programs.
Kadima's founder and CEO, Prof. David Feifel, MD PHD, has been a pioneer and international thought leader for advanced interventional treatment of psychiatric disorders such as depression, anxiety, PTSD and related disorders for more than three decades. Among other things, he is a co-author on a recent landmark expert consensus paper for treating depression with TMS, endorsed by three leading organizations in the field (ref). He also established the first clinical program to use subanesthetic dose ketamine infusions for neuropsychiatric disorders at UC San Diego, where he is currently Professor Emeritus of Psychiatry. His 150 peer-reviewed publications and several patents have provided global thought leadership on advanced approaches to treating psychiatric conditions, including integration of medicines like ketamine with neuromodulation such as, Transcranial Magnetic Stimulation and Digital Therapeutics. Kadima's experience will guide the growing network of NRXP HOPE Therapeutics clinics to an integrated, multi-modal approach to treating suicidal depression, anxiety and PTSD that is far more effective than ketamine alone.
More on TelAve News
Upon consummation of the proposed acquisition, Dr. Feifel will serve as NRXP HOPE's Chief Medical Innovation Officer (CMIO), focused on identifying and evaluating new developments in the treatment of CNS disorders and insuring Hope clinics are at the forefront of interventional psychiatry delivery, and leading global clinical trials to continue to advance the ability to treat these lethal diseases.
Dr. Feifel will join NRXP Chairman, Prof. Jonathan Javitt in presenting a Keynote address at the 8th Annual Neuroscience Innovation Forum during the JP Morgan Healthcare Conference in San Francisco, CA on the 12th of January 2025, and will join in meeting investors over the following week.
Initial Section of U.S. New Drug Application to the FDA for NRX-100 (IV Ketamine) for the Treatment of Suicidal Depression
On December 30th NRXP announced the transmission of first section of its New Drug Application (NDA) for NRX-100 (ketamine) for electronic filing with the U.S. Food & Drug Administration (FDA). NRX-100 was initially granted Fast Track Designation in 2017 for use in combination with NRX-101 (D-cycloserine/lurasidone) for treatment of suicidal bipolar depression. The Company is now seeking to expand the indication to include Suicidal Ideation in Major Depressive Disorder and other forms of depression, based on data from NIH- and European Government-funded trials that have been summarized on the NRXP website.
While assembly of the clinical data sections is being completed, FDA has asked the Company to submit the 1800-page manufacturing section (Module 3) of the NDA to enable immediate review prior to submission of final efficacy data and other sections of the NDA expected in the first quarter of 2025.
The NRx presentation of ketamine differs from the form of ketamine used in anesthesia in that it contains no potentially toxic preservatives and utilizes diversion-resistant packaging to enhance the traceability of a medicine known to have abuse potential.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
Disclaimer and Disclosure: https://corporateads.com/disclaimer/
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Kadima Neuropsychiatry Institute Targeted as First Acquisition for HOPE Subsidiary International Network of Interventional Psychiatry Clinics.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research.
Completion of NDA Filing Expected in First Quarter of 2025
Plans to Participate in J.P. Morgan Healthcare Conference on January 13-16, 2025, in San Francisco, CA.
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. $NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
Kadima Neuropsychiatry Institute Expected First Acquisition for HOPE Subsidiary International Network of Interventional Psychiatry Clinics
More on TelAve News
- Trump's Executive Order Rescheduling Cannabis: Accelerating M&A in a Multibillion-Dollar Industry
- Genuine Hospitality, LLC Selected to Operate Hilton Garden Inn Birmingham SE / Liberty Park
- Documentary "Prescription for Violence: Psychiatry's Deadly Side Effects" Premieres, Exposes Link Between Psychiatric Drugs and Acts of Mass Violence
- Price Improvement on Luxurious Lāna'i Townhome with Stunning Ocean Views
- Nextvisit Co-Founder Ryan Yannelli Identifies Six Critical Factors for Behavioral Health Providers Evaluating AI Scribes in 2026
On January 2nd NRXP announced the planned acquisition of the Kadima Neuropsychiatry Institute of La Jolla, CA, per the previously announced Letter of Intent, for the Company's HOPE subsidiary network. Kadima is expected to serve as the flagship clinic for HOPE's planned international network of interventional psychiatry clinics, designed to provide advanced treatments for debilitating diseases such as depression, anxiety and PTSD.
Kadima is one of the world's premier interventional psychiatry clinics and was among the first to introduce Ketamine Therapy for Central Nervous System (CNS) disorders at scale in the clinic setting. The clinic offers a full range of cutting-edge treatments for suicidal depression, anxiety, Post Traumatic Stress Disorder (PTSD) and other CNS disorders. Those treatment options include Ketamine Therapy, Spravato® (nasal esketamine), Transcranial Magnetic Stimulation as well as medication management.
Kadima also has a robust research division and is a leading investigative site for innovative CNS treatments, specializing in psychedelic research, for which it has served as a leading site in nearly all major clinical trials in this area. Kadima has contracts in place with the US Department of Veterans Affairs and also treats active-duty military personnel in the US Department of Defense under Tricare and other treatment programs.
Kadima's founder and CEO, Prof. David Feifel, MD PHD, has been a pioneer and international thought leader for advanced interventional treatment of psychiatric disorders such as depression, anxiety, PTSD and related disorders for more than three decades. Among other things, he is a co-author on a recent landmark expert consensus paper for treating depression with TMS, endorsed by three leading organizations in the field (ref). He also established the first clinical program to use subanesthetic dose ketamine infusions for neuropsychiatric disorders at UC San Diego, where he is currently Professor Emeritus of Psychiatry. His 150 peer-reviewed publications and several patents have provided global thought leadership on advanced approaches to treating psychiatric conditions, including integration of medicines like ketamine with neuromodulation such as, Transcranial Magnetic Stimulation and Digital Therapeutics. Kadima's experience will guide the growing network of NRXP HOPE Therapeutics clinics to an integrated, multi-modal approach to treating suicidal depression, anxiety and PTSD that is far more effective than ketamine alone.
More on TelAve News
- CredHub and Real Property Management Join Forces to Empower Franchise Owners with Rental Payment Credit Reporting Solutions
- Leimert Park Announces Weeklong Kwanzaa Festival & Kwanzaa Parade Celebrating Black History, Culture, and Community
- Renowned Alternative Medicine Specialist Dr. Sebi and His African Bio Mineral Balance Therapy Are the Focus of New Book
- Psychiatric Drug Damage Ignored for Decades; CCHR Demands Federal Action
- Why Millions Are Losing Sexual Sensation, And Why It's Not Age, Hormones, or Desire
Upon consummation of the proposed acquisition, Dr. Feifel will serve as NRXP HOPE's Chief Medical Innovation Officer (CMIO), focused on identifying and evaluating new developments in the treatment of CNS disorders and insuring Hope clinics are at the forefront of interventional psychiatry delivery, and leading global clinical trials to continue to advance the ability to treat these lethal diseases.
Dr. Feifel will join NRXP Chairman, Prof. Jonathan Javitt in presenting a Keynote address at the 8th Annual Neuroscience Innovation Forum during the JP Morgan Healthcare Conference in San Francisco, CA on the 12th of January 2025, and will join in meeting investors over the following week.
Initial Section of U.S. New Drug Application to the FDA for NRX-100 (IV Ketamine) for the Treatment of Suicidal Depression
On December 30th NRXP announced the transmission of first section of its New Drug Application (NDA) for NRX-100 (ketamine) for electronic filing with the U.S. Food & Drug Administration (FDA). NRX-100 was initially granted Fast Track Designation in 2017 for use in combination with NRX-101 (D-cycloserine/lurasidone) for treatment of suicidal bipolar depression. The Company is now seeking to expand the indication to include Suicidal Ideation in Major Depressive Disorder and other forms of depression, based on data from NIH- and European Government-funded trials that have been summarized on the NRXP website.
While assembly of the clinical data sections is being completed, FDA has asked the Company to submit the 1800-page manufacturing section (Module 3) of the NDA to enable immediate review prior to submission of final efficacy data and other sections of the NDA expected in the first quarter of 2025.
The NRx presentation of ketamine differs from the form of ketamine used in anesthesia in that it contains no potentially toxic preservatives and utilizes diversion-resistant packaging to enhance the traceability of a medicine known to have abuse potential.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
Disclaimer and Disclosure: https://corporateads.com/disclaimer/
Source: Corporate Ads
0 Comments
Latest on TelAve News
- TRIO Heating, Air & Plumbing Now Ranks #1 in San Jose
- Milwaukee Job Corps Center Hosts Alumni Day, Calls Alumni to Action on Open Enrollment Campaign
- Golden Paper Identifies Global Growth in Packaging Papers and Upgrades Its High-End Production Capacity
- Champagne, Caviar Bumps & Pole Performances — Welcome the New Year Early with HandPicked Social Club
- A New Soul Album: Heart Of Kwanzaa, 7-Day Celebration
- Allegiant Management Group Named 2025 Market Leader in Orlando by PropertyManagement.com
- NAFMNP Awarded USDA Cooperative Agreement to Continue MarketLink Program Under FFAB
- Costa Oil - 10 Minute Oil Change Surpasses 70 Locations with Construction of San Antonio, TX Stores — Eyes Growth Via Acquisition or Being Acquired
- LaTerra and Respark Under Contract with AIMCO to Acquire a $455M, 7-Property Chicago Multifamily Portfolio
- Record Revenue, Tax Tailwinds, and AI-Driven Scale: Why Off The Hook YS Inc. Is Emerging as a Standout in the $57 Billion U.S. Marine Market
- VSee Health (N A S D A Q: VSEE) Secures $6.0M At-Market Investment, Accelerates Expansion as Revenues Surge
- Children Rising Appoints Marshelle A. Wilburn as New Executive Director
- Fairmint CEO Joris Delanoue Elected General Director of the Canton Foundation
- Sleep Basil Mattress Co.'s Debuts New Home Page Showcasing Performance Sleep Solutions for Active Denver Lifestyles
- Bent Danholm Joins The American Dream TV as Central Florida Host
- The Nature of Miracles Celebrates 20th Anniversary Third Edition Published by DreamMakers Enterprises LLC
- Artificial Intelligence Leader Releases Children's Book on Veterans Day
- Felicia Allen Hits #1 Posthumously with "Christmas Means Worship"
- CCHR Documentary Probes Growing Evidence Linking Psychiatric Drugs to Violence
- Creative Investment Research Warns AT&T Rollback Undermines Market Integrity