Popular on TelAve
- Still Using Ice? FrostSkin Reinvents Hydration
- Nest Finders Property Management Named #1 in Jacksonville and Ranked #99 Nationwide
- OneVizion Announces Next Phase of Growth as Brad Kitchens Joins Board of Directors
- Half of Finnish Online Gambling Expenditure Now Flows to Offshore Instant Casinos as License Applications Open March 1, 2026
- Market Value Enhancement From 2 Important New US Patents Issued for Strengthening Hair Enzyme Booster Technology to Caring Brands (NAS DAQ: CABR)
- EPP Pricing Platform announces leadership transition to support long-term growth and continuity
- Luxury Lake-View Home Launches in Kissimmee's Bellalago community, Offering Privacy, Space, and Florida Resort-Style Living
- Ice Melts. Infrastructure Fails. What Happens to Clean Water?
- RTC Communications Completes Next Level Connect Fiber Expansion Bringing Multi-Gig Broadband to West Boggs Community
- Cold. Clean. Anywhere. Meet FrostSkin
Similar on TelAve
- Progressive Dental & The Closing Institute Partner with Zest Dental Solutions to Elevate Full-Arch Growth and Patient Outcomes
- National Expansion Ignited Across Amazon $AMZN, Chewy $CHWY & Walmart $WMT: NDT Pharmaceuticals, Inc. (Stock Symbol: NDTP) $NDTP
- Tarrytown Expocare Pharmacy Announces Strategic Leadership Appointments to Accelerate Growth and Innovation
- NaturismRE Launches the NRE Health Institute to Advance Evidence-Informed Public Health Research
- Progressive Dental & The Closing Institute Partner with Zest Dental Solutions to Elevate Full-Arch Growth and Patient Outcomes
- CCHR: While Damaging Antipsychotics Win Approval, Proven Non-Drug Alternatives Remain Ignored
- At 25, She Became One of the Youngest AAPI Female Founders to Win One of the World's Most Prestigious Design Awards for a Lamp That Makes You Smile
- VENUS Goes Live on CATEX Exchange As UK Financial Ltd Activates The Premier Division Of The Maya Meme's League
- Special Alert: Undervalued Opportunity: IQSTEL (N A S D A Q: IQST) Positioned for Explosive Multi-Year Growth
- Triple-Digit Growth, Strategic N A S D A Q Uplist, Plus A Scalable Healthcare Rollout Model: Stock Symbol: CDIX
High-Impact Mental Health Platform Approaching a Defining Regulatory Moment: Eclipsing 70,000 Patients on Real World Use of Ketamine: N ASDAQ: NRXP
TelAve News/10885896
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP has Entered into a Joint Offering with neurocare Group for Neuroplastic Therapy Targeting Depression, PTSD and Other Mental Health Afflictions
MIAMI - TelAve -- As the mental health crisis deepens in the United States, few biotech companies are as directly aligned with both urgent unmet medical need and near-term regulatory catalysts as NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP). With groundbreaking real-world data from over 70,000 patients, multiple FDA pathways underway, a newly debt-free balance sheet, and an expanding neuroplastic therapy ecosystem, NRXP is positioning itself as a potentially transformative force in the treatment of suicidal depression, bipolar depression, PTSD, and chronic pain.
A Massive, Unmet Need with No Approved Drug Solution
According to the CDC, more than 13 million Americans seriously consider suicide each year, yet no medication is currently FDA-approved to treat suicidal ideation. Today, electroconvulsive therapy (ECT) remains the only approved intervention—an invasive option often reserved as a last resort.
NRXP is attempting to change that paradigm.
The company is advancing NRX-100, a preservative-free intravenous ketamine, under FDA Fast Track designation for the treatment of suicidal depression and bipolar depression. Importantly, NRXP recently licensed Real World Evidence (RWE) data from over 70,000 U.S. patients, marking one of the largest datasets ever assembled for ketamine use in suicidality.
70,000-Patient Ketamine Dataset Headed to the FDA
On January 14, NRXP announced plans to submit this expansive real-world dataset to the FDA in support of Accelerated Approval of NRX-100.
Preliminary analysis of a 20,000-patient subset revealed:
The full 70,000-patient analysis will be presented to regulators, strengthening NRXP's case that ketamine—when delivered in a safer, preservative-free formulation—may finally offer a pharmacologic option for acute suicidality.
More on TelAve News
If successful, NRXP could help bring the first FDA-approved drug for suicidal ideation to market.
KETAFREE™: A Cleaner Ketamine with a Clear Regulatory Path
Parallel to NRX-100, NRXP is pursuing approval of KETAFREE™, a preservative-free IV ketamine via an Abbreviated New Drug Application (ANDA). In December, the FDA confirmed the ANDA is "substantially complete" and assigned a PDUFA goal date of July 29, 2026.
Why this matters:
Approval of KETAFREE™ could establish NRXP as a differentiated supplier in a large, existing market—separate from the novel drug opportunity represented by NRX-100.
NRX-101: A Breakthrough Therapy with Expanding Potential
NRXP's flagship pipeline asset, NRX-101, is an FDA-designated Breakthrough Therapy for suicidal treatment-resistant bipolar depression. The oral combination drug (D-cycloserine + lurasidone) was specifically engineered to deliver neuroplastic benefits while mitigating hallucination risk—an innovation protected by composition-of-matter patents worldwide.
In late 2024, NRXP expanded NRX-101's potential by adding a new pipeline indication: augmentation of Transcranial Magnetic Stimulation (TMS).
Recent real-world and clinical data suggest:
With projections that over 1 million Americans per year may receive TMS by 2030, this new indication opens a previously unanticipated commercial pathway for NRX-101.
Building a Neuroplastic Therapy Ecosystem with neurocare
In January, NRXP announced a joint initiative with neurocare Group AG to create a nationwide network of integrated neuroplastic therapy clinics targeting depression, PTSD, and other serious mental health disorders.
More on TelAve News
The model combines:
The rollout will leverage:
Early pilot programs—particularly among first responders with PTSD and depression—have demonstrated exceptionally high remission rates, positioning NRXP at the intersection of drug development and scalable clinical delivery.
A Clean Balance Sheet and Analyst Validation
In December, NRXP eliminated 100% of its balance-sheet debt, converting $5.4 million into equity with no additional warrants—a notable reset that strengthens the company's financial footing ahead of key regulatory milestones.
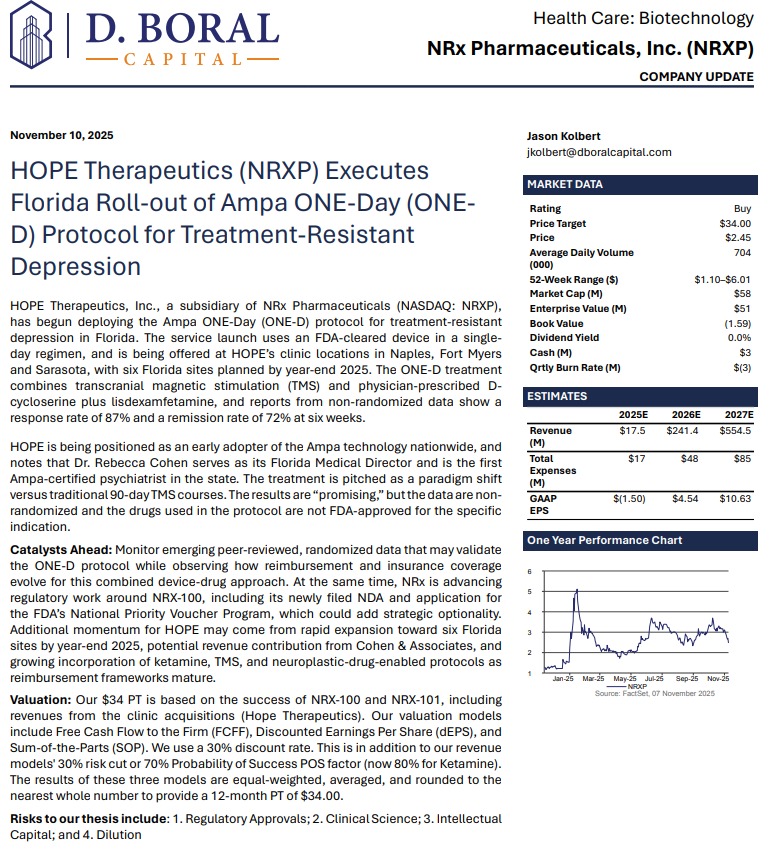
Adding to investor confidence, D. Boral Capital issued a Buy rating with a $34 price target, citing NRXP's pipeline breadth, regulatory momentum, and differentiated mental health strategy.
The Bottom Line
NRx Pharmaceuticals stands out in a crowded biotech landscape by targeting:
As regulatory submissions advance and clinical integration expands, NRXP may be approaching an inflection point—one with the potential to redefine how suicidal depression and treatment-resistant mental illness are treated in the U.S.
Ticker: N A S D A Q: NRXP
More Information:
For more information:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Media Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com | Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
A Massive, Unmet Need with No Approved Drug Solution
According to the CDC, more than 13 million Americans seriously consider suicide each year, yet no medication is currently FDA-approved to treat suicidal ideation. Today, electroconvulsive therapy (ECT) remains the only approved intervention—an invasive option often reserved as a last resort.
NRXP is attempting to change that paradigm.
The company is advancing NRX-100, a preservative-free intravenous ketamine, under FDA Fast Track designation for the treatment of suicidal depression and bipolar depression. Importantly, NRXP recently licensed Real World Evidence (RWE) data from over 70,000 U.S. patients, marking one of the largest datasets ever assembled for ketamine use in suicidality.
70,000-Patient Ketamine Dataset Headed to the FDA
On January 14, NRXP announced plans to submit this expansive real-world dataset to the FDA in support of Accelerated Approval of NRX-100.
Preliminary analysis of a 20,000-patient subset revealed:
- Rapid resolution of depression and suicidality
- Clinical responses consistent with prior randomized NIH-sponsored trials
- Outcomes that compare favorably to currently approved antidepressant products
The full 70,000-patient analysis will be presented to regulators, strengthening NRXP's case that ketamine—when delivered in a safer, preservative-free formulation—may finally offer a pharmacologic option for acute suicidality.
More on TelAve News
- National Expansion Ignited Across Amazon $AMZN, Chewy $CHWY & Walmart $WMT: NDT Pharmaceuticals, Inc. (Stock Symbol: NDTP) $NDTP
- Distributed Social Media - Own Your Content
- Tarrytown Expocare Pharmacy Announces Strategic Leadership Appointments to Accelerate Growth and Innovation
- New Environmental Thriller "The Star Thrower" Reimagines a Classic Lesson in Individual Impact
- Summit Appoints Javier Cabeza as Data, AI, and Analytics Practice Lead
If successful, NRXP could help bring the first FDA-approved drug for suicidal ideation to market.
KETAFREE™: A Cleaner Ketamine with a Clear Regulatory Path
Parallel to NRX-100, NRXP is pursuing approval of KETAFREE™, a preservative-free IV ketamine via an Abbreviated New Drug Application (ANDA). In December, the FDA confirmed the ANDA is "substantially complete" and assigned a PDUFA goal date of July 29, 2026.
Why this matters:
- Current ketamine products contain benzethonium chloride (BZT), a preservative not recognized as safe by the FDA
- KETAFREE™ eliminates this additive
- The global ketamine market is estimated at $750 million annually
- Manufactured in the U.S., aligning with MAHA initiatives to remove toxic substances from medicines and strengthen domestic supply chains
Approval of KETAFREE™ could establish NRXP as a differentiated supplier in a large, existing market—separate from the novel drug opportunity represented by NRX-100.
NRX-101: A Breakthrough Therapy with Expanding Potential
NRXP's flagship pipeline asset, NRX-101, is an FDA-designated Breakthrough Therapy for suicidal treatment-resistant bipolar depression. The oral combination drug (D-cycloserine + lurasidone) was specifically engineered to deliver neuroplastic benefits while mitigating hallucination risk—an innovation protected by composition-of-matter patents worldwide.
In late 2024, NRXP expanded NRX-101's potential by adding a new pipeline indication: augmentation of Transcranial Magnetic Stimulation (TMS).
Recent real-world and clinical data suggest:
- 87% clinical response
- 72% remission
- Achieved after a single day of TMS combined with oral D-cycloserine
With projections that over 1 million Americans per year may receive TMS by 2030, this new indication opens a previously unanticipated commercial pathway for NRX-101.
Building a Neuroplastic Therapy Ecosystem with neurocare
In January, NRXP announced a joint initiative with neurocare Group AG to create a nationwide network of integrated neuroplastic therapy clinics targeting depression, PTSD, and other serious mental health disorders.
More on TelAve News
- March Is Skiing's Smartest Buying Window
- Cancun Airport Transportation Expands Fleet Ahead of Record Passenger Growth at Cancun International Airport
- Tobu Group's "T-home Series" of Accommodations in Tokyo Just Opened "T-home KEI."
- Custom Wooden Token Manufacturer Celebrates 10 Years of Helping Brands Stay Top of Mind
- NaturismRE Launches the NRE Health Institute to Advance Evidence-Informed Public Health Research
The model combines:
- TMS
- Ketamine and other neuroplastic drugs
- Hyperbaric oxygen therapy
- Psychotherapy
The rollout will leverage:
- neurocare's existing clinic footprint
- HOPE Therapeutics clinics
- 400+ Apollo® TMS machines already deployed nationwide
Early pilot programs—particularly among first responders with PTSD and depression—have demonstrated exceptionally high remission rates, positioning NRXP at the intersection of drug development and scalable clinical delivery.
A Clean Balance Sheet and Analyst Validation
In December, NRXP eliminated 100% of its balance-sheet debt, converting $5.4 million into equity with no additional warrants—a notable reset that strengthens the company's financial footing ahead of key regulatory milestones.
Adding to investor confidence, D. Boral Capital issued a Buy rating with a $34 price target, citing NRXP's pipeline breadth, regulatory momentum, and differentiated mental health strategy.
The Bottom Line
NRx Pharmaceuticals stands out in a crowded biotech landscape by targeting:
- One of the most urgent unmet needs in medicine
- With unprecedented real-world clinical data
- Multiple FDA pathways (Fast Track, ANDA, Breakthrough Therapy)
- A growing neuroplastic therapy infrastructure
- And a now debt-free balance sheet
As regulatory submissions advance and clinical integration expands, NRXP may be approaching an inflection point—one with the potential to redefine how suicidal depression and treatment-resistant mental illness are treated in the U.S.
Ticker: N A S D A Q: NRXP
More Information:
For more information:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Media Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com | Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: CorporateAds
0 Comments
Latest on TelAve News
- Vesica Health Receives FDA Breakthrough Device Designation for AssureMDx
- Lineus Medical's SafeBreak® Vascular Added to Alliant GPO Contract
- Cancun All Inclusive is ready for Spring Break 2026 with new Resorts, Exclusive Deals, activities and more!
- 66% of US Bankruptcies Are Medical — So Americans Are Building Businesses That Cover Healthcare Emergencies
- Ludex Partners With Certified Trading Card Association (CTCA) To Elevate Standards And Innovation In The Trading Card Industry
- Best Book Publishing Company for Aspiring Authors
- Dr. Nadene Rose Releases Moving Memoir on Faith, Grief, and Divine Presence
- Gigasoft Solves AI's Biggest Charting Code Problem: Hallucinated Property Names
- ASTI Ignites the Space Economy: Powering SpaceX's NOVI AI Pathfinder with Breakthrough Solar Technology: Ascent Solar Technologies (N A S D A Q: ASTI)
- Hiring has reached a "Digital Stalemate"—Now, an ex-Google recruiter is giving candidates the answers
- 2026 Pre-Season Testing Confirms a Two-Tier Grid as Energy Management Defines Formula 1's New Era
- Platinum Car Audio LLC Focuses on Customer-Driven Vehicle Audio and Electronics Solutions
- Postmortem Pathology Expands Independent Autopsy Services in Kansas City
- Postmortem Pathology Expands Independent Autopsy Services Across Colorado
- $38 Million in U.S. Government Contract Awards Secured Through Strategic Partner. Establishing Multi-Year Defense Revenue Platform Through 2032: $BLIS
- Mecpow M1: A Safe & Affordable Laser Engraver Built for Home DIY Beginners
- CrashStory.com Launches First Colorado Crash Data Platform Built for Victims, Not Lawyers
- Inkdnylon Earns BBB Accreditation for Verified Business Integrity
- Josh Stout "The Western Project"
- Open House Momentum Builds at Heritage at South Brunswick