Popular on TelAve
- Still Using Ice? FrostSkin Reinvents Hydration
- OneVizion Announces Next Phase of Growth as Brad Kitchens Joins Board of Directors
- Nest Finders Property Management Named #1 in Jacksonville and Ranked #99 Nationwide
- Inkdnylon Simplifies Digitizing and Vector Art Nationwide With Clear Pricing and Guided File Support
- Half of Finnish Online Gambling Expenditure Now Flows to Offshore Instant Casinos as License Applications Open March 1, 2026
- Market Value Enhancement From 2 Important New US Patents Issued for Strengthening Hair Enzyme Booster Technology to Caring Brands (NAS DAQ: CABR)
- Georgia's Lanier Islands Resort Tees Up for a New Era of Golf in Spring 2026
- Kaltra Expands Microchannel Innovation to Deliver Lower Refrigerant Charge
- EPP Pricing Platform announces leadership transition to support long-term growth and continuity
- purelyIV Expands Concierge Wellness Platform with New IV Therapies, Memberships, and Digital Experience
Similar on TelAve
- Special Alert: Undervalued Opportunity: IQSTEL (N A S D A Q: IQST) Positioned for Explosive Multi-Year Growth
- Triple-Digit Growth, Strategic N A S D A Q Uplist, Plus A Scalable Healthcare Rollout Model: Stock Symbol: CDIX
- Vesica Health Receives FDA Breakthrough Device Designation for AssureMDx
- Lineus Medical's SafeBreak® Vascular Added to Alliant GPO Contract
- 66% of US Bankruptcies Are Medical — So Americans Are Building Businesses That Cover Healthcare Emergencies
- Ludex Partners With Certified Trading Card Association (CTCA) To Elevate Standards And Innovation In The Trading Card Industry
- Gigasoft Solves AI's Biggest Charting Code Problem: Hallucinated Property Names
- ASTI Ignites the Space Economy: Powering SpaceX's NOVI AI Pathfinder with Breakthrough Solar Technology: Ascent Solar Technologies (N A S D A Q: ASTI)
- Hiring has reached a "Digital Stalemate"—Now, an ex-Google recruiter is giving candidates the answers
- Platinum Car Audio LLC Focuses on Customer-Driven Vehicle Audio and Electronics Solutions
OncoBeta announces the expansion of Rhenium-SCT for non-melanoma skin cancer patients in Italy
TelAve News/10836504
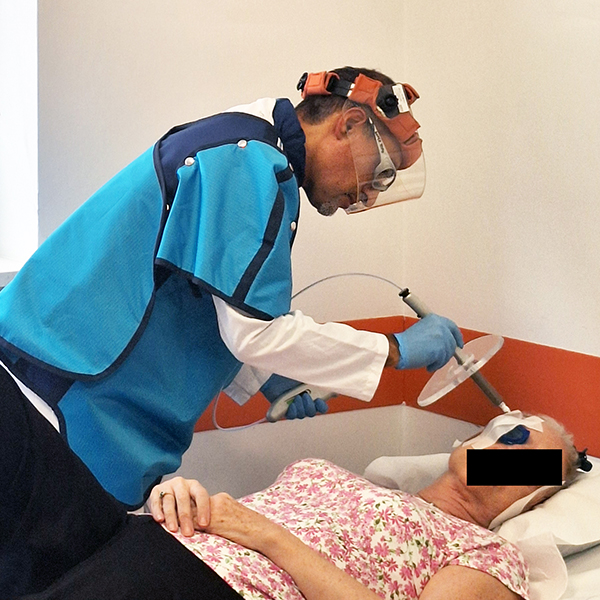
BOZEN, Italy - TelAve -- OncoBeta®, a leading medical device company specialising in innovative epidermal radioisotope therapies, has announced the expansion of Rhenium-SCT® (Skin Cancer Therapy) treatment services in Italy with the Bolzano Hospital the latest to come on board. This marks a significant advancement in the availability of non-melanoma skin cancer (NMSC) treatment options within the country.
Rhenium skin cancer therapy is an advanced radionuclide therapy that offers a painless,*1,2 single-session,†1-3 non-invasive‡1 treatment without disfiguring scarring.1,4 Treatment with Rhenium-SCT employs a superficial application of a paste containing β-emitting particles directly over the NMSC lesion, targeting cancer cells without the need for surgery.1,4
Dr. Mohsen Farsad at Bolzano Hospital has been impressed with the Rhenium-SCT device, noting, "I have witnessed impressive outcomes with several patients treated here in Italy already, and am pleased with the design of the device technology."
"Rhenium skin cancer therapy fulfills many patients' desires for a non-surgical alternative for the treatment of NMSCs especially in relation to difficult localisations. I am pleased to continue treating suitable patients with Rhenium-SCT", adds Dr. Farsad.
Shannon D. Brown III, CCO at OncoBeta, says, "While surgical intervention remains the standard-of-care for NMSC treatment, it often leads to suboptimal outcomes. Rhenium-SCT offers a non-surgical alternative with a simple application directly to the area needed to treat, and allows patients to resume their daily activities with minimal disruption."
According to GLOBOCAN estimates, the number of new NMSC cases in Italy is projected to rise from 29,000 to 41,700 between 2022 and 2045.5 Epidemiological data for Europe shows an annual incidence rate of NMSCs at 129.3 cases per 100,000 men and 90.8 per 100,000 women. Basal cell carcinoma (BCC) accounts for approximately 15% of all diagnosed cancers in Italy, with an annual incidence of around 100 cases per 100,000 inhabitants.6
Dr. Gerhard Dahlhoff, Medical Director at OncoBeta, emphasised, "Rhenium-SCT enables targeted, non-invasive treatment of NMSCs while preserving adjacent healthy tissue. This patient-friendly approach underscores our commitment to enhancing treatment options for those affected by NMSCs."
More on TelAve News
Rhenium-SCT is currently available in Germany, Austria, Switzerland, Italy, Spain, United Kingdom, South Africa and Australia. Rhenium-SCT will be provided to patients at Bolzano Hospital by OncoBeta's distribution partner, DSD Pharma.
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.1 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.1,4
The Rhenium-SCT is a painless,*1,2 single session,†1-3 non-invasive‡1 therapy that provides aesthetic results, even in cases otherwise considered difficult to treat.1,4,7 The Rhenium-SCT utilizes the radioisotope rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalized therapy that is only applied to the area needed to treat without affecting the healthy tissue.4,7 The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Most cases of NMSCs (basal cell carcinomas and squamous cell carcinomas) can be treated using the Rhenium-SCT in one single session. †1-4 Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.1,7,8
About OncoBeta®
OncoBeta® with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting NMSCs. OncoBeta has perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards.
Find out more about the Rhenium-SCT at www.oncobeta.com
Follow us on social media:
More on TelAve News
LinkedIn: https://www.linkedin.com/company/oncobeta-gmbh/
Facebook: https://www.facebook.com/oncobeta/
Instagram: https://www.instagram.com/oncobeta_gmbh/
About DSD Pharma
DSD Pharma is a distributor for diagnostics, theranostics and radiopharmaceutical/medical devices in the field of nuclear medicine and radiopharmaceuticals. DSD Pharma offers a product portfolio developed together at the forefront of research to its customers. This ensures the safety of latest technologies for the HCP and the best available diagnosis/theranosis/therapy for the patient.
Find out more about DSD Pharma at www.dsd-pharma.com
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties and other factors, many of which are outside of OncoBeta's control and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No reported pain during procedure.1,2
†Complete tumour regression in 98.5% of lesions treated.3
‡A treatment is considered non-invasive when no cut or break in the skin is created.9
References
Rhenium skin cancer therapy is an advanced radionuclide therapy that offers a painless,*1,2 single-session,†1-3 non-invasive‡1 treatment without disfiguring scarring.1,4 Treatment with Rhenium-SCT employs a superficial application of a paste containing β-emitting particles directly over the NMSC lesion, targeting cancer cells without the need for surgery.1,4
Dr. Mohsen Farsad at Bolzano Hospital has been impressed with the Rhenium-SCT device, noting, "I have witnessed impressive outcomes with several patients treated here in Italy already, and am pleased with the design of the device technology."
"Rhenium skin cancer therapy fulfills many patients' desires for a non-surgical alternative for the treatment of NMSCs especially in relation to difficult localisations. I am pleased to continue treating suitable patients with Rhenium-SCT", adds Dr. Farsad.
Shannon D. Brown III, CCO at OncoBeta, says, "While surgical intervention remains the standard-of-care for NMSC treatment, it often leads to suboptimal outcomes. Rhenium-SCT offers a non-surgical alternative with a simple application directly to the area needed to treat, and allows patients to resume their daily activities with minimal disruption."
According to GLOBOCAN estimates, the number of new NMSC cases in Italy is projected to rise from 29,000 to 41,700 between 2022 and 2045.5 Epidemiological data for Europe shows an annual incidence rate of NMSCs at 129.3 cases per 100,000 men and 90.8 per 100,000 women. Basal cell carcinoma (BCC) accounts for approximately 15% of all diagnosed cancers in Italy, with an annual incidence of around 100 cases per 100,000 inhabitants.6
Dr. Gerhard Dahlhoff, Medical Director at OncoBeta, emphasised, "Rhenium-SCT enables targeted, non-invasive treatment of NMSCs while preserving adjacent healthy tissue. This patient-friendly approach underscores our commitment to enhancing treatment options for those affected by NMSCs."
More on TelAve News
- Lineus Medical's SafeBreak® Vascular Added to Alliant GPO Contract
- Cancun All Inclusive is ready for Spring Break 2026 with new Resorts, Exclusive Deals, activities and more!
- 66% of US Bankruptcies Are Medical — So Americans Are Building Businesses That Cover Healthcare Emergencies
- Ludex Partners With Certified Trading Card Association (CTCA) To Elevate Standards And Innovation In The Trading Card Industry
- Best Book Publishing Company for Aspiring Authors
Rhenium-SCT is currently available in Germany, Austria, Switzerland, Italy, Spain, United Kingdom, South Africa and Australia. Rhenium-SCT will be provided to patients at Bolzano Hospital by OncoBeta's distribution partner, DSD Pharma.
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.1 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.1,4
The Rhenium-SCT is a painless,*1,2 single session,†1-3 non-invasive‡1 therapy that provides aesthetic results, even in cases otherwise considered difficult to treat.1,4,7 The Rhenium-SCT utilizes the radioisotope rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalized therapy that is only applied to the area needed to treat without affecting the healthy tissue.4,7 The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Most cases of NMSCs (basal cell carcinomas and squamous cell carcinomas) can be treated using the Rhenium-SCT in one single session. †1-4 Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.1,7,8
About OncoBeta®
OncoBeta® with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting NMSCs. OncoBeta has perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards.
Find out more about the Rhenium-SCT at www.oncobeta.com
Follow us on social media:
More on TelAve News
- Dr. Nadene Rose Releases Moving Memoir on Faith, Grief, and Divine Presence
- Gigasoft Solves AI's Biggest Charting Code Problem: Hallucinated Property Names
- ASTI Ignites the Space Economy: Powering SpaceX's NOVI AI Pathfinder with Breakthrough Solar Technology: Ascent Solar Technologies (N A S D A Q: ASTI)
- Hiring has reached a "Digital Stalemate"—Now, an ex-Google recruiter is giving candidates the answers
- 2026 Pre-Season Testing Confirms a Two-Tier Grid as Energy Management Defines Formula 1's New Era
LinkedIn: https://www.linkedin.com/company/oncobeta-gmbh/
Facebook: https://www.facebook.com/oncobeta/
Instagram: https://www.instagram.com/oncobeta_gmbh/
About DSD Pharma
DSD Pharma is a distributor for diagnostics, theranostics and radiopharmaceutical/medical devices in the field of nuclear medicine and radiopharmaceuticals. DSD Pharma offers a product portfolio developed together at the forefront of research to its customers. This ensures the safety of latest technologies for the HCP and the best available diagnosis/theranosis/therapy for the patient.
Find out more about DSD Pharma at www.dsd-pharma.com
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties and other factors, many of which are outside of OncoBeta's control and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No reported pain during procedure.1,2
†Complete tumour regression in 98.5% of lesions treated.3
‡A treatment is considered non-invasive when no cut or break in the skin is created.9
References
- Castellucci P, et al. Eur J Nucl Med Mol Imaging. 2021; 48: 1511–1521.
- Cipriani C, et al. J Dermatol Treat. 2022;33(2):969–975. Epub 22 Jul 2020.
- Cipriani C, et al. In Therapeutic Nuclear Medicine. 2014. RP Baum (Ed), New York: Springer.
- Tietze JK, et al. Clin Nucl Med. 2023;48:869–76.
- Global Burden of Disease Cancer Collaboration, et al. JAMA Oncol. 2019;5(12):1749-1768.
- Sordi E, et al. Epidemiologia. 2024;5:1–10.
- Cipriani C, et al. International J Nucl Med. 2017; July:114–112.
- Sedda AF, et al. Clin Exp Dermatol. 2008;33(6):745–749.
- Australian Therapeutic Goods Administration. Acronym and glossary of terms. Available at: https://www.tga.gov.au/resources/acronyms-and-glossary-terms. (Accessed June 2024).
Source: OncoBeta GmbH
0 Comments
Latest on TelAve News
- IDpack v4 Launches: A Major Evolution in Cloud-Based ID Card Issuance
- CCHR Says Psychiatry's Admission on Antidepressant Withdrawal Comes Far Too Late
- 505 Plumbing, Heating & Cooling Launches in Albuquerque, Bringing a Customer-First Approach to Home Services
- As AI.com Sells For Record $70 Million, Attention Now Turns To ArtificialIntelligence.com
- ClearBeam Networks Launches HomeStation: Home Phone 2.0
- AOW Event Sponsored By The Stanglwirt Resort a renowned five-star Austrian wellness destination
- Average US gambler spends $210 per month in 2026
- 10X Recruitment Launches Operator-Led Executive Search for Behavioral Health and Legal Leaders
- Integris Composites developing armor for military in Arctic Circle
- Caraline Skincare's Gentle Glow Cleansing Oil Named Finalist for Best Face Cleanser at the 2026 CertClean Clean Beauty Awards
- Workplace safety ideas from the front lines to highlight Applied Ergonomics Conference in Arlington, Texas
- OpenSSL Corporation Advisory Committees' Elections 2026: Results Announcement
- Zarova Vodka Expands Its Ultra-Premium Spirits Portfolio Through Strategic Acquisitions
- The Legal AI Showdown: Westlaw, Lexis, ChatGPT… or EvenSteven?
- François Arnaud, star of Heated Rivalry, is the real-life inspiration behind Christopher Stoddard's novel At Night Only
- UK Financial Ltd Sets February 27 CATEX Debut for VENUS Coin, Opening Limited Early Access Through MayaPro Wallet
- Ice Melts. Infrastructure Fails. What Happens to Clean Water?
- Delay In Federal Disaster Assistance Causing Failure Of Small Business In Disaster Areas
- Capsadyn® Launches on Amazon, Offering Non-Burning Capsaicin Pain Relief
- When Representation No Longer Reflects the District — Why I'm Voting for Pete Verbica